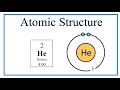
Helium and Noble Gas Properties
Interactive Video
•
Chemistry
•
6th - 8th Grade
•
Hard
Emma Peterson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What model is used to visualize the atomic structure of helium?
Quantum model
Thomson model
Bohr model
Rutherford model
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the atomic number of helium indicate?
Number of energy levels
Number of neutrons
Number of electrons
Number of protons
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons does a neutral helium atom have?
Four
Three
Two
One
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In which energy level are helium's electrons placed?
First energy level
Third energy level
Fourth energy level
Second energy level
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is helium considered a noble gas?
It has a full outer shell with two electrons
It is highly reactive
It has eight valence electrons
It can form multiple bonds
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What makes helium unique among the noble gases?
It is the heaviest noble gas
It has a full outer shell with eight electrons
It only needs two electrons for a full outer shell
It can easily gain electrons
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the first energy level being full for helium?
It makes helium reactive
It classifies helium as a noble gas
It means helium has more than two electrons
It allows helium to bond easily
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Physical and Chemical Properties
Quiz
•
8th Grade

20 questions
Counting Atoms Practice
Quiz
•
8th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

40 questions
PBA #1 Test Review 2025 version
Quiz
•
7th Grade

20 questions
Chemical Reactions
Quiz
•
8th Grade

32 questions
Counting atoms, Balancing, Law of Conservation of Mass Review
Quiz
•
8th Grade

20 questions
States of Matter
Quiz
•
8th Grade

22 questions
matter review
Quiz
•
6th Grade