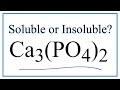
Solubility of Calcium Phosphate
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Jackson Turner
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is calcium phosphate?
Molecular
Covalent
Ionic
Metallic
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, which group of elements generally forms soluble phosphates?
Noble gases
Group 1 elements
Transition metals
Group 2 elements
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is calcium phosphate considered insoluble in water based on solubility rules?
It is a covalent compound
It reacts with water to form a new compound
It forms a gas when dissolved
It does not fall under the exceptions for soluble phosphates
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the solubility chart indicate about calcium phosphate?
It is highly soluble
It is slightly soluble
It forms a precipitate
It is insoluble
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the solubility chart confirm the solubility rules for calcium phosphate?
It does not list calcium phosphate
It shows calcium phosphate as a gas
It shows calcium phosphate as insoluble
It shows calcium phosphate as soluble
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to calcium phosphate when placed in water?
It reacts violently
It dissolves completely
It remains mostly solid
It forms a gas
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is noted about the small amount of calcium phosphate that might dissolve in water?
It changes the color of the solution
It forms a new compound
It is negligible and can be ignored
It is significant and should be considered
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Electron Configurations and Periodic Trends
Interactive video
•
9th - 10th Grade

6 questions
Understanding Problem-Solving Strategies
Interactive video
•
9th - 12th Grade

11 questions
Properties and Reactions of Halogens
Interactive video
•
8th - 10th Grade

8 questions
Trends in Atomic Radii
Interactive video
•
9th - 10th Grade

10 questions
Percent Composition and Molecular Structure
Interactive video
•
9th - 10th Grade

11 questions
Types of Chemical Bonds
Interactive video
•
9th - 10th Grade

11 questions
Oxidation Numbers and States
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Equations and Ions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

10 questions
Identifying Types of Chemical Reactions
Interactive video
•
6th - 10th Grade