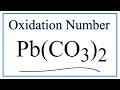
Oxidation States in PbCO3
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of a neutral compound like PbCO3?
Positive
Negative
Zero
Depends on the elements
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can't the oxidation state of lead be determined just by looking at Pb in PbCO3?
Lead is a noble gas
Lead is a non-metal
Lead is a transition metal
Lead is a halogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many carbonate ions are present in PbCO3?
Four
One
Two
Three
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the ionic charge of the carbonate ion in PbCO3?
3-
4-
2-
1-
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What must the oxidation state of lead be in PbCO3 to ensure the compound is neutral?
+5
+4
+3
+2
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total oxidation number for the two carbonate ions in PbCO3?
-2
-6
-8
-4
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where can you find additional help for determining the oxidation state of carbon in the carbonate ion?
In the glossary
In the appendix
In the textbook
In the video description
Similar Resources on Wayground

6 questions
Oxidation States and Chlorine Compounds
Interactive video
•
9th - 10th Grade

8 questions
Zinc Compounds and Oxidation States
Interactive video
•
9th - 10th Grade

6 questions
Oxidation States and Neutral Compounds
Interactive video
•
9th - 10th Grade

8 questions
Lead Compounds and Carbonate Ions
Interactive video
•
9th - 10th Grade

8 questions
Balancing Chemical Equations
Interactive video
•
9th - 10th Grade

6 questions
Tin and Carbonate Compounds Concepts
Interactive video
•
9th - 10th Grade

8 questions
Oxidation Numbers and Ionic Charges
Interactive video
•
9th - 10th Grade

6 questions
Nickel Oxidation States and Charges
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade