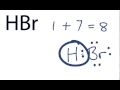
Hydrogen and Bromine Valence Electrons
Interactive Video
•
Chemistry
•
8th - 9th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which group does Hydrogen belong to on the periodic table?
Group 17
Group 1
Group 2
Group 7
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does Bromine have?
1
5
8
7
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons used in the Lewis structure of HBr?
2
6
4
8
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does Hydrogen need for a full outer shell?
2
8
1
4
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the number 8 in the context of Bromine's valence electrons?
It is the number of protons in Bromine.
It represents the number of bonds Bromine can form.
It is the total number of electrons in Bromine's outer shell.
It is the atomic number of Bromine.
Similar Resources on Wayground

6 questions
Valence Electrons in SiCl4
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Stability in BH3
Interactive video
•
9th - 10th Grade

6 questions
BrF3 Lewis Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons in IBr2- Structure
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons in NBr3
Interactive video
•
9th - 10th Grade

6 questions
Cl2O Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
SF2 Lewis Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons in Chemistry
Interactive video
•
8th - 9th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

20 questions
Quick Check #1 2025-Pure Substances and Mixtures
Quiz
•
8th Grade

14 questions
Ice breaker
Lesson
•
9th - 12th Grade