What type of ion is carbonate considered to be?
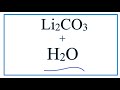
Carbonate Chemistry Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Monatomic ion
Polyatomic ion
Cation
Anion
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why are carbonates generally considered insoluble in water?
They form strong covalent bonds.
They are nonpolar molecules.
They tend to form precipitates.
They react violently with water.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does 'ss' indicate in a solubility table?
Strongly soluble
Slightly soluble
Solid state
Saturated solution
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of a solubility table in this context?
To measure pH levels
To calculate molarity
To determine solubility
To predict reaction speed
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When lithium carbonate dissolves in water, what ions are formed?
Li+ and CO2 3-
Li- and CO3+
Li2+ and CO3-
Li+ and CO3 2-
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is a coefficient of 2 placed in front of Li in the equation?
To balance the charge
To show complete dissolution
To account for two lithium ions
To indicate a solid state
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the subscript '2' in Li2CO3?
It means the compound is highly reactive.
It shows the compound is a gas.
It suggests the compound is a liquid.
It indicates two lithium atoms are present.
Create a free account and access millions of resources
Similar Resources on Quizizz

11 questions
Lead(II) Carbonate Properties and Reactions
Interactive video
•
9th - 10th Grade

7 questions
Solubility and Lithium Compounds
Interactive video
•
9th - 10th Grade

7 questions
Lithium Carbonate Compound Concepts
Interactive video
•
9th - 10th Grade

8 questions
Oxidation Numbers in Compounds
Interactive video
•
9th - 10th Grade

10 questions
Ionic Compounds and Lithium Chemistry
Interactive video
•
9th - 10th Grade

9 questions
Balancing Chemical Equations with Polyatomic Ions
Interactive video
•
9th - 10th Grade

9 questions
Solubility and Reactions of Metal Oxides
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Ammonium Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Quizizz

15 questions
Multiplication Facts
Quiz
•
4th Grade

25 questions
SS Combined Advisory Quiz
Quiz
•
6th - 8th Grade

40 questions
Week 4 Student In Class Practice Set
Quiz
•
9th - 12th Grade

40 questions
SOL: ILE DNA Tech, Gen, Evol 2025
Quiz
•
9th - 12th Grade

20 questions
NC Universities (R2H)
Quiz
•
9th - 12th Grade

15 questions
June Review Quiz
Quiz
•
Professional Development

20 questions
Congruent and Similar Triangles
Quiz
•
8th Grade

25 questions
Triangle Inequalities
Quiz
•
10th - 12th Grade
Discover more resources for Chemistry

40 questions
Week 4 Student In Class Practice Set
Quiz
•
9th - 12th Grade

40 questions
SOL: ILE DNA Tech, Gen, Evol 2025
Quiz
•
9th - 12th Grade

20 questions
NC Universities (R2H)
Quiz
•
9th - 12th Grade

25 questions
Triangle Inequalities
Quiz
•
10th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade

24 questions
LSO - Virus, Bacteria, Classification - sol review 2025
Quiz
•
9th Grade

65 questions
MegaQuiz v2 2025
Quiz
•
9th - 12th Grade

10 questions
GPA Lesson
Lesson
•
9th - 12th Grade