
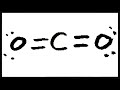
Understanding Lewis Structures for CO2
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard

Jennifer Brown
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in drawing the Lewis structure for CO2?
Calculate the total number of valence electrons
Write the correct skeletal structure
Form double or triple bonds
Distribute electrons among atoms
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can't hydrogen be a central atom in a Lewis structure?
It is always a noble gas
It has too many valence electrons
It can only form one bond
It is too electronegative
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is typically placed in the central position of a molecule?
The atom with the highest atomic number
The atom with the most valence electrons
The least electronegative atom
The most electronegative atom
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you determine the number of valence electrons for main group elements?
By their atomic number
By their period number
By their electronegativity
By their group number
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in CO2?
12
16
18
20
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in distributing electrons among atoms in CO2?
Place lone pairs on the central atom
Calculate the total number of electron pairs
Place two electrons between each pair of atoms
Form double bonds
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When distributing electrons, which atoms should receive lone pairs first?
The atoms with the fewest bonds
The most electronegative atoms
The least electronegative atoms
The central atom
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

10 questions
Probability Practice
Quiz
•
4th Grade

15 questions
Probability on Number LIne
Quiz
•
4th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

6 questions
Appropriate Chromebook Usage
Lesson
•
7th Grade

10 questions
Greek Bases tele and phon
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Predicting Products
Quiz
•
9th - 12th Grade

11 questions
Balancing Chemical Equations
Lesson
•
9th Grade

10 questions
Exploring Types of Chemical Reactions
Interactive video
•
6th - 10th Grade

19 questions
Stoichiometry, % yield, Limiting Reactants
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Ionic and Covalent Bonding Concepts
Interactive video
•
6th - 10th Grade

7 questions
GCSE Chemistry - Balancing Chemical Equations #4
Interactive video
•
9th - 10th Grade

12 questions
Percent Yield
Quiz
•
10th Grade