
14 Q
9th - 12th
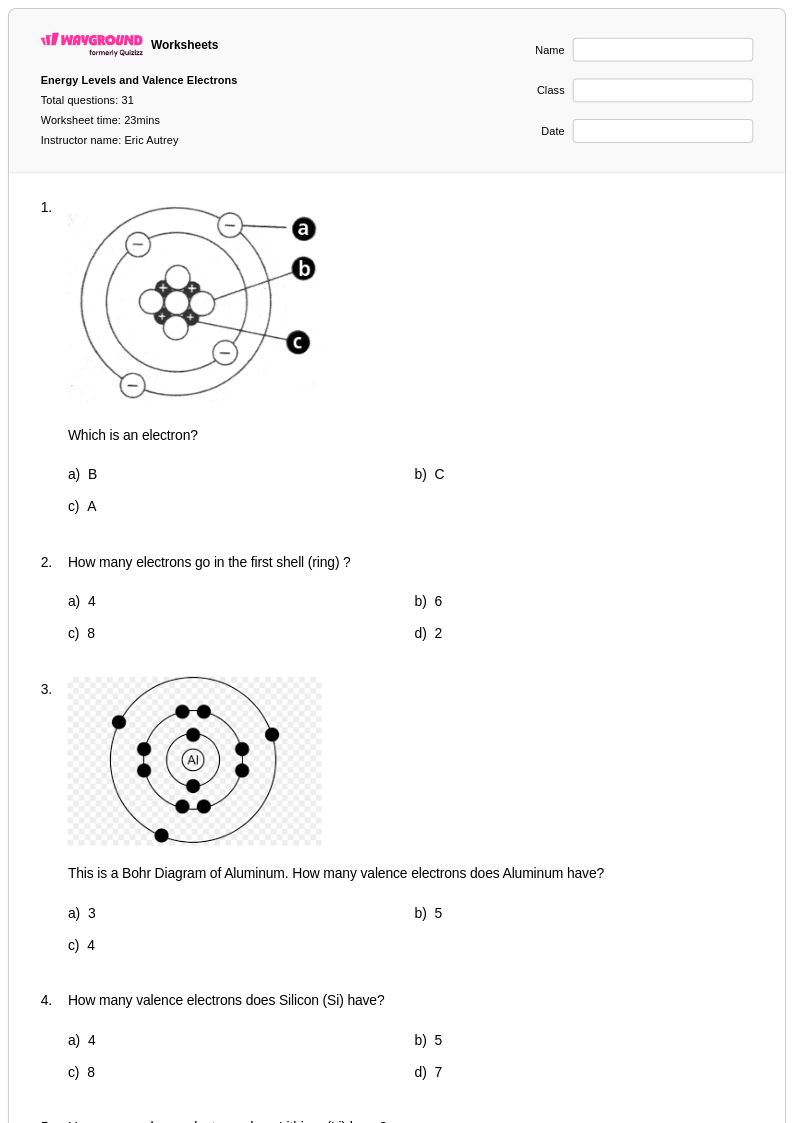
31 Q
9th - 12th

50 Q
12th
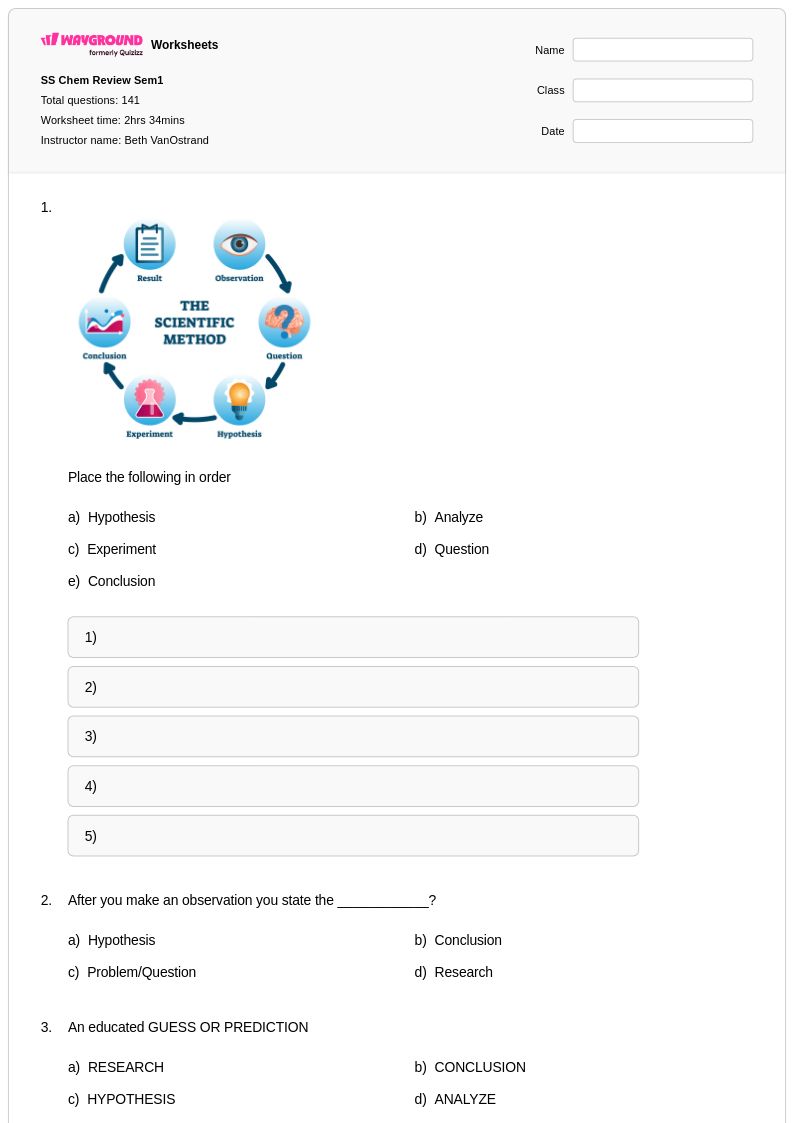
141 Q
9th - 12th

45 Q
9th - 12th
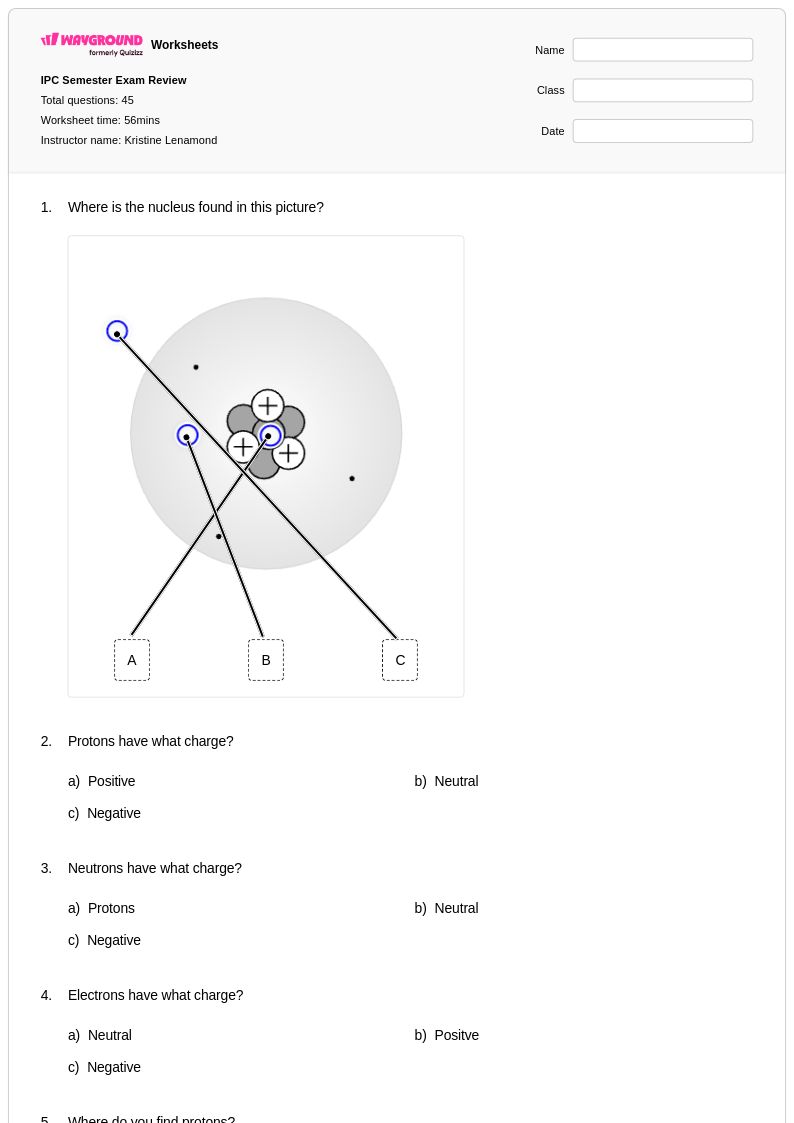
45 Q
9th - 12th
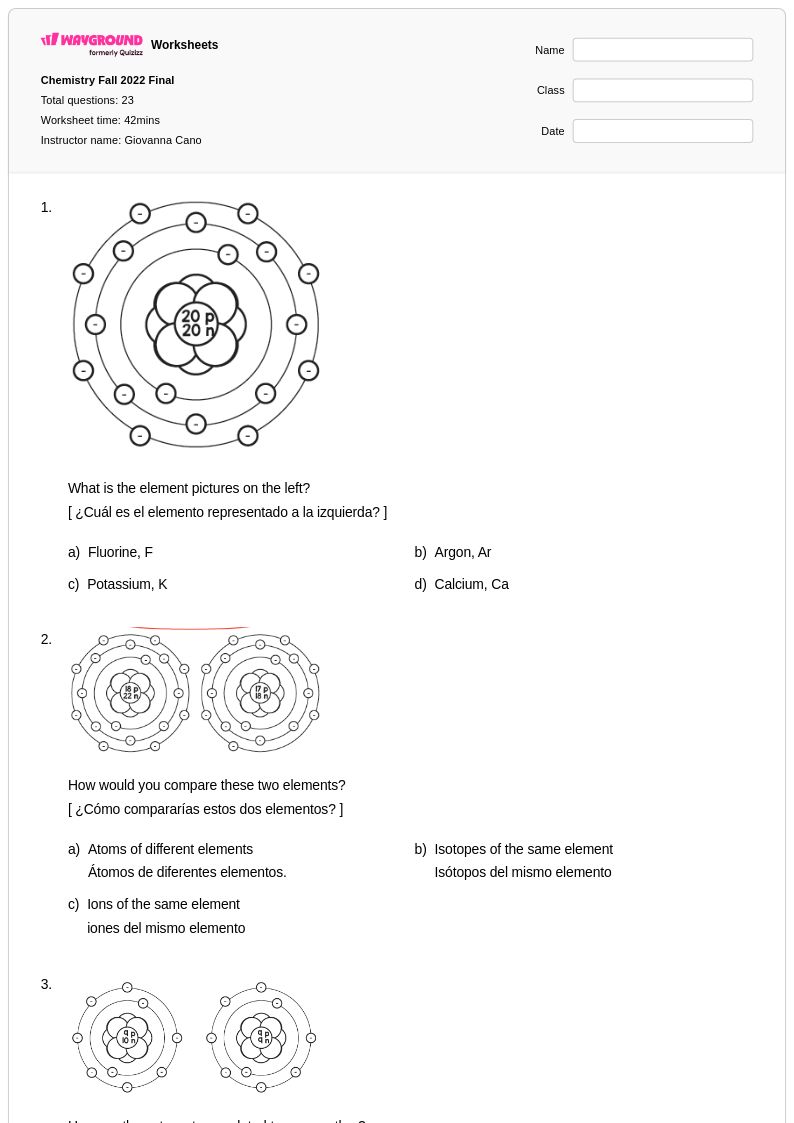
23 Q
12th

16 Q
8th - 12th
20 Q
9th - Uni
15 Q
10th - Uni
20 Q
12th
13 Q
9th - 12th
18 Q
11th - Uni
21 Q
9th - 12th
25 Q
8th - Uni

10 Q
9th - 12th

115 Q
9th - Uni
36 Q
9th - 12th
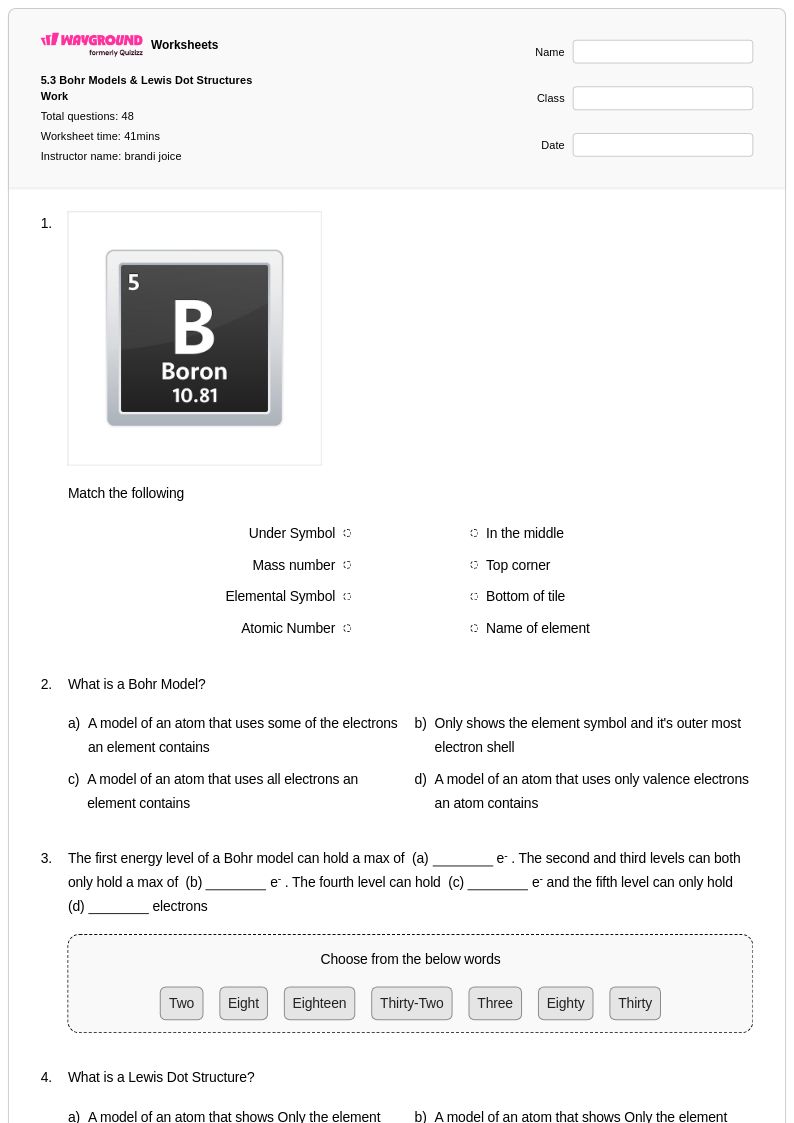
48 Q
9th - 12th
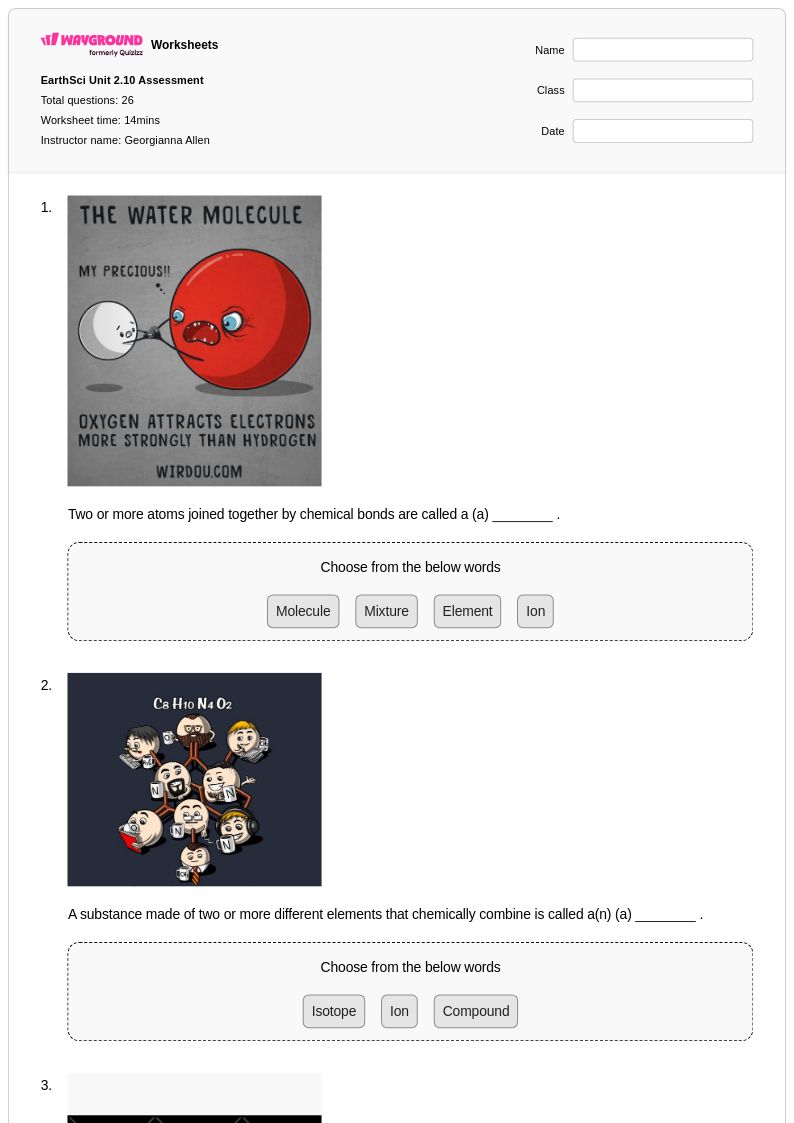
26 Q
9th - Uni
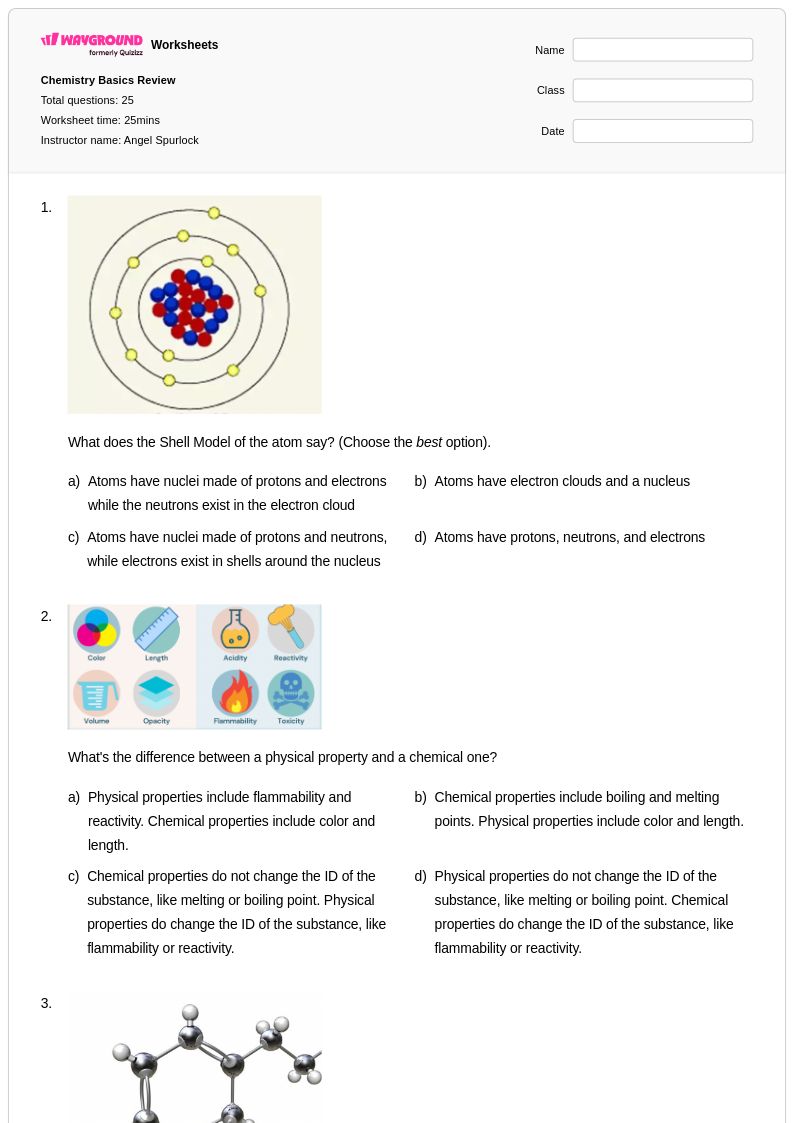
25 Q
9th - 12th

22 Q
12th
32 Q
9th - 12th

5 Q
9th - 12th
Explore Other Subject Worksheets for class 12
Explore printable Atomic Bonding worksheets for Class 12
Atomic bonding worksheets for Class 12 students available through Wayground (formerly Quizizz) provide comprehensive coverage of the fundamental forces that hold atoms together in chemical compounds. These expertly designed practice problems strengthen students' understanding of ionic, covalent, and metallic bonding mechanisms, helping them master concepts such as electronegativity differences, Lewis structures, VSEPR theory, and molecular geometry. Each worksheet collection includes detailed answer keys that guide students through complex bonding scenarios, from predicting bond polarity to drawing accurate molecular representations. The free printables and pdf resources offer extensive practice with real-world applications, enabling students to connect theoretical bonding principles with observable chemical properties and molecular behavior.
Wayground (formerly Quizizz) empowers educators with millions of teacher-created atomic bonding resources that streamline lesson planning and enhance student learning outcomes. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific chemistry standards and learning objectives, while differentiation tools enable customization for diverse student needs and skill levels. Available in both printable pdf format and interactive digital versions, these resources support flexible classroom implementation for remediation, enrichment, and regular skill practice. Teachers can modify existing worksheets or combine multiple resources to create targeted assignments that address individual student gaps in understanding complex bonding theories, molecular structures, and chemical compound formation.
