
40Q
10th - Uni

15Q
11th - Uni

34Q
12th
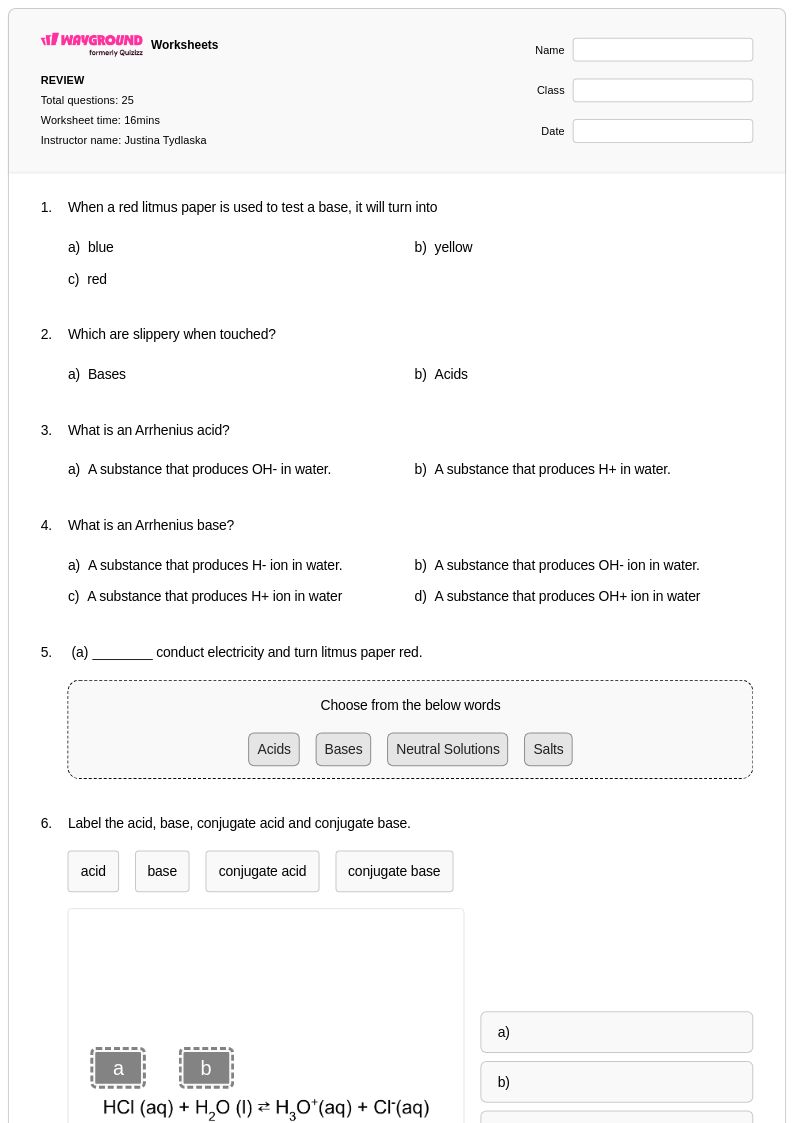
25Q
9th - 12th

100Q
12th

14Q
9th - Uni
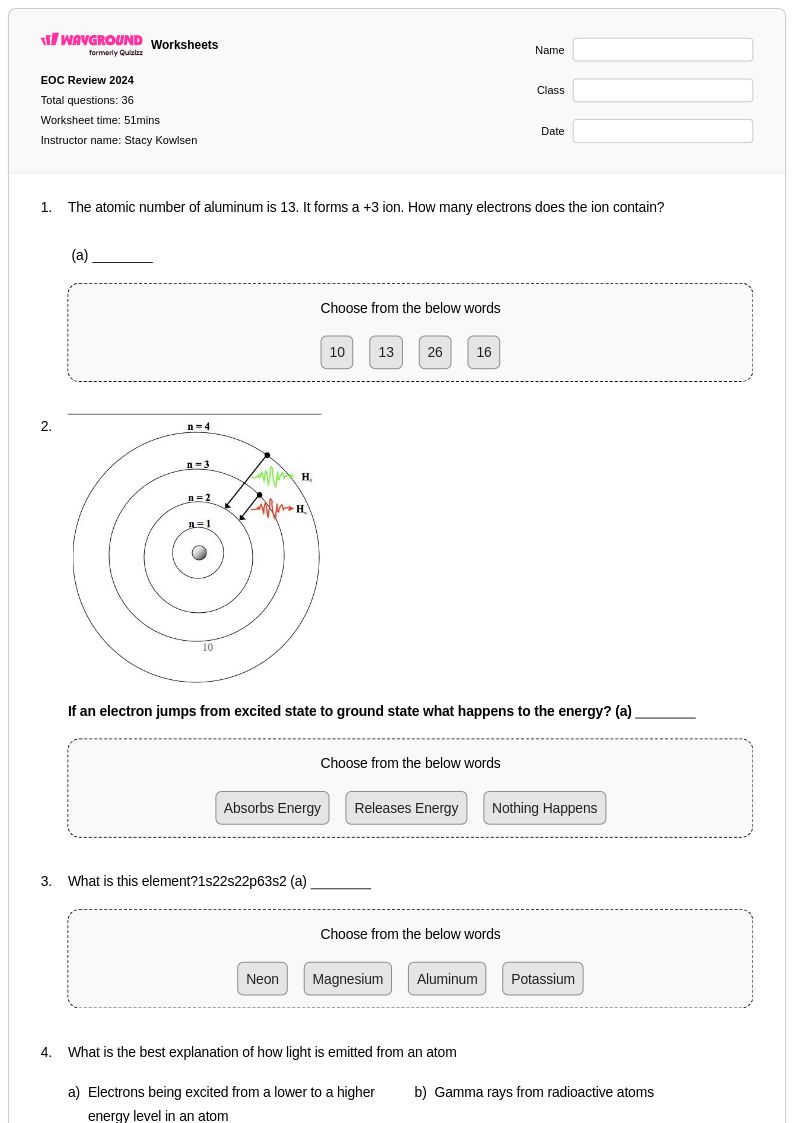
36Q
9th - 12th

21Q
12th
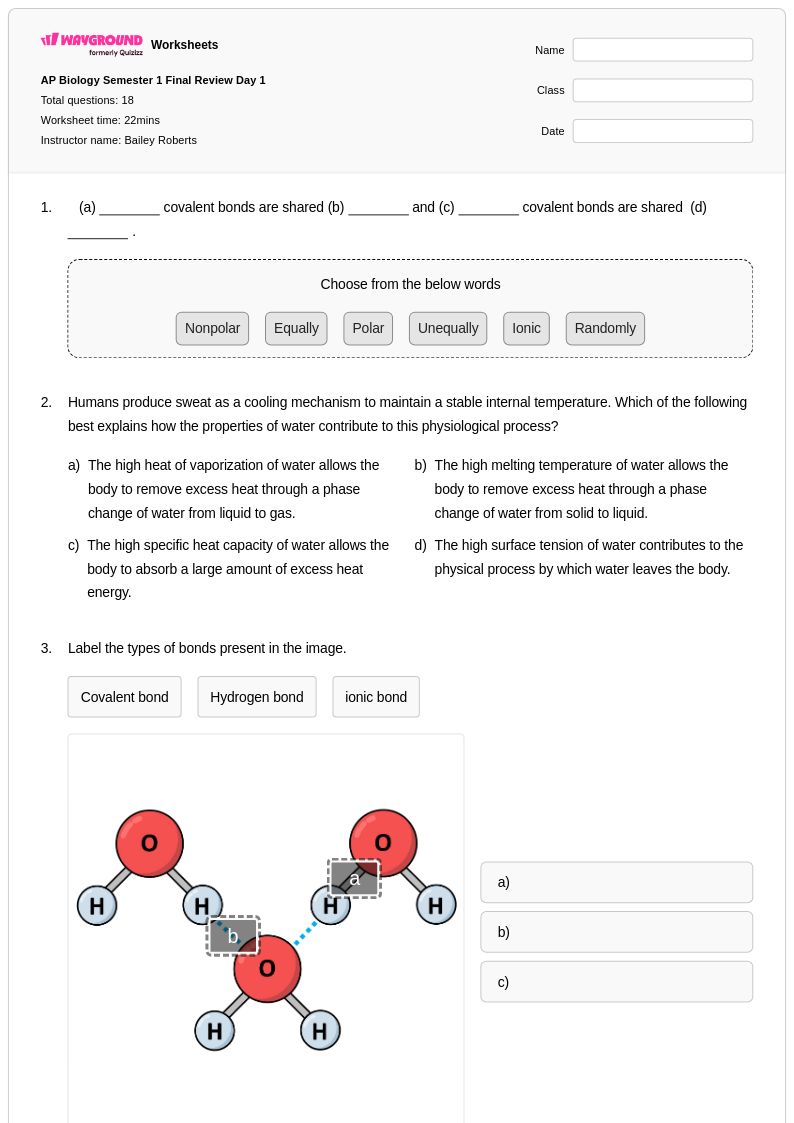
18Q
12th
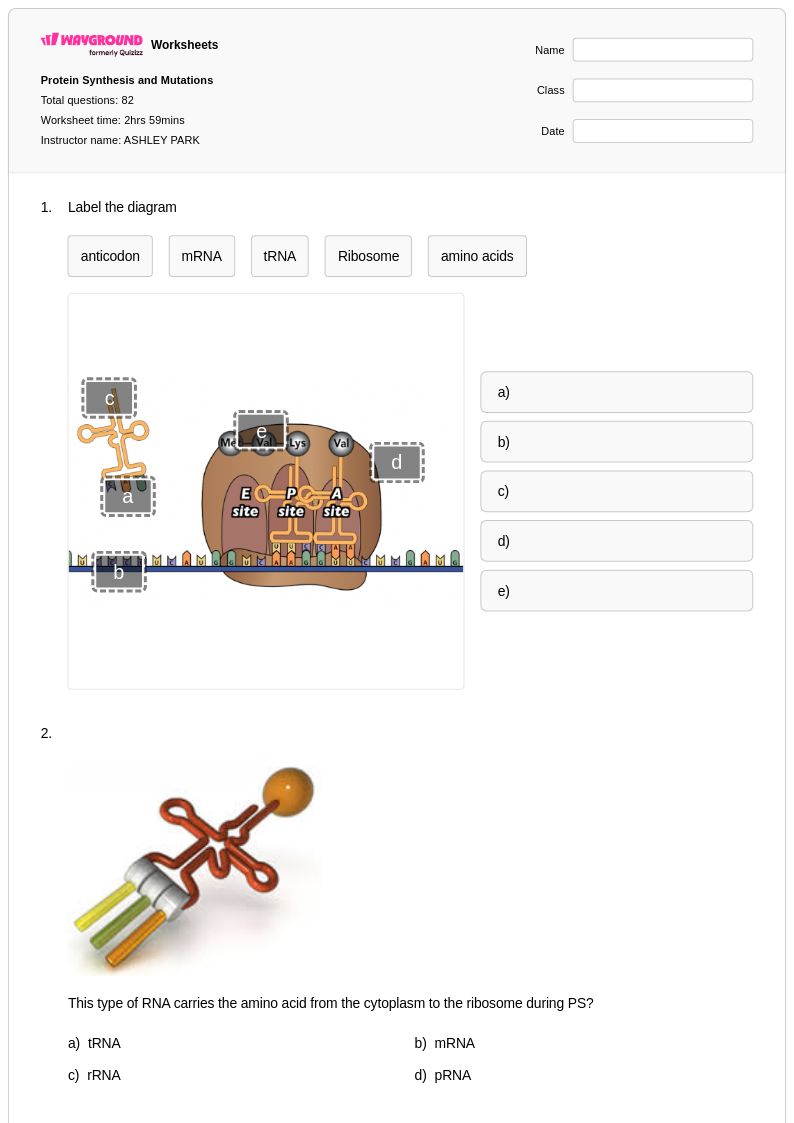
82Q
9th - 12th

20Q
10th - Uni
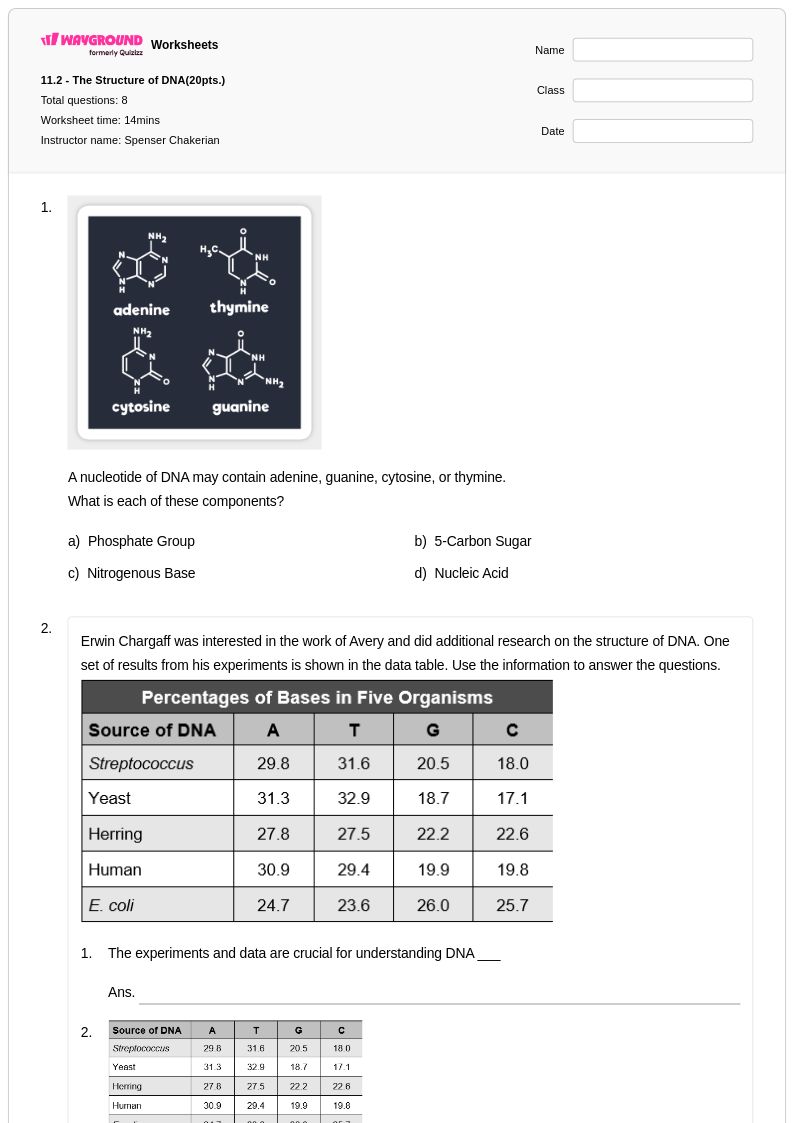
12Q
9th - 12th
64Q
9th - 12th

35Q
9th - 12th

20Q
12th

23Q
9th - 12th

36Q
11th - Uni
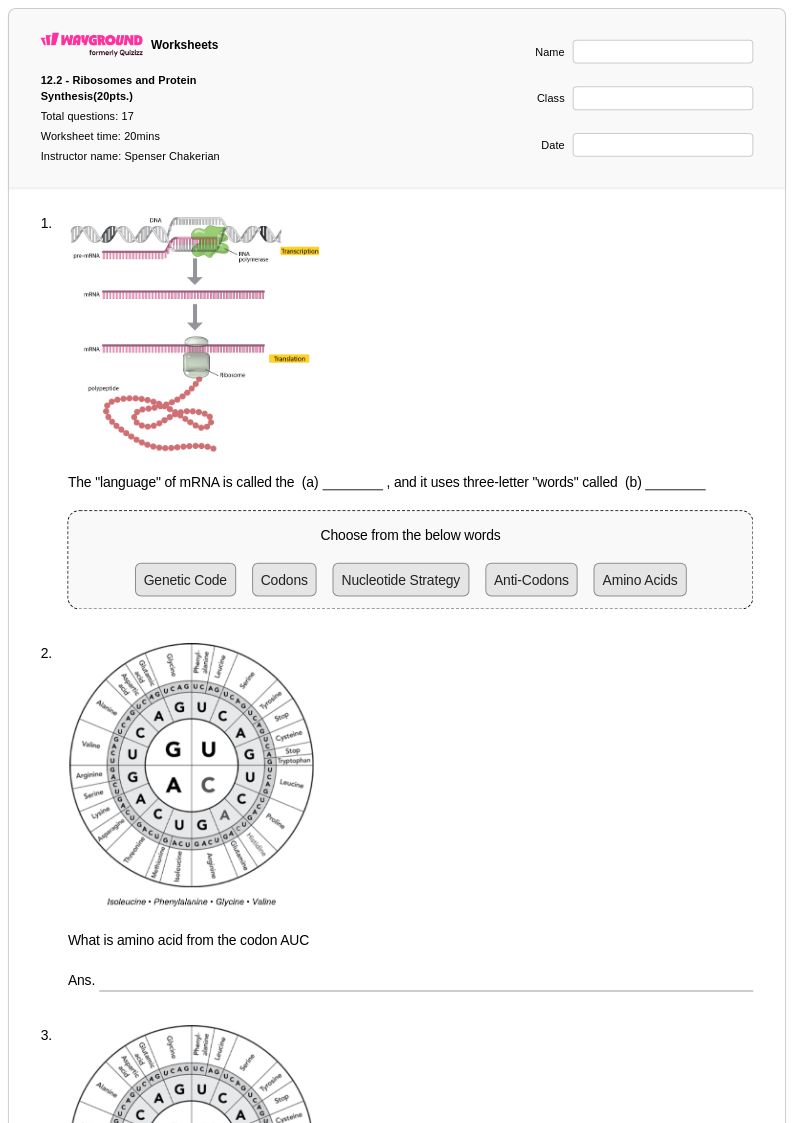
17Q
9th - 12th

19Q
10th - 12th

15Q
9th - 12th

17Q
8th - Uni
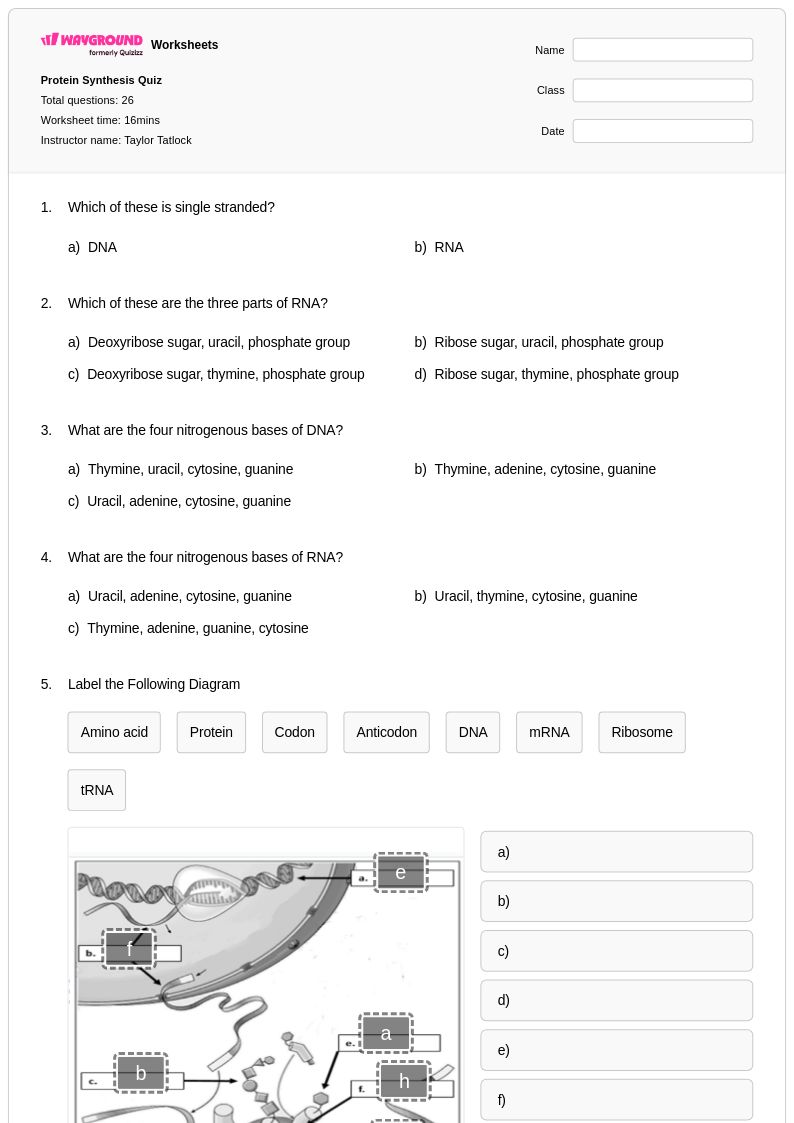
26Q
12th
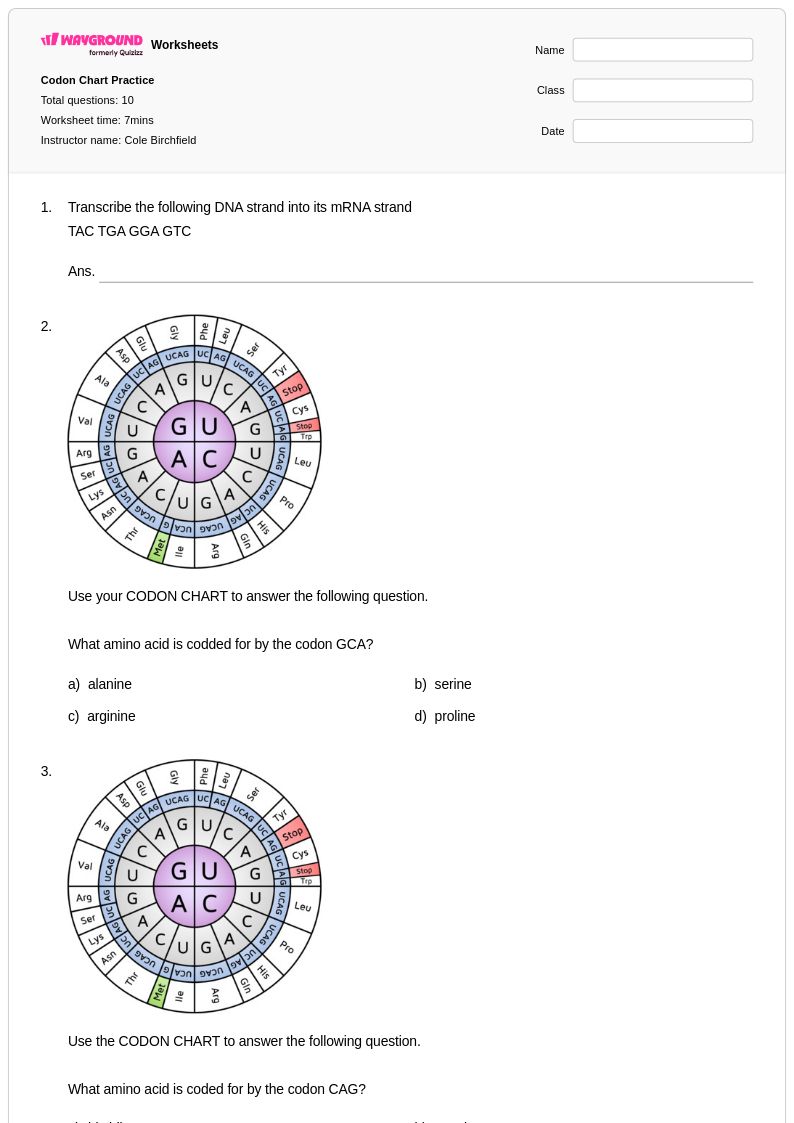
10Q
9th - 12th
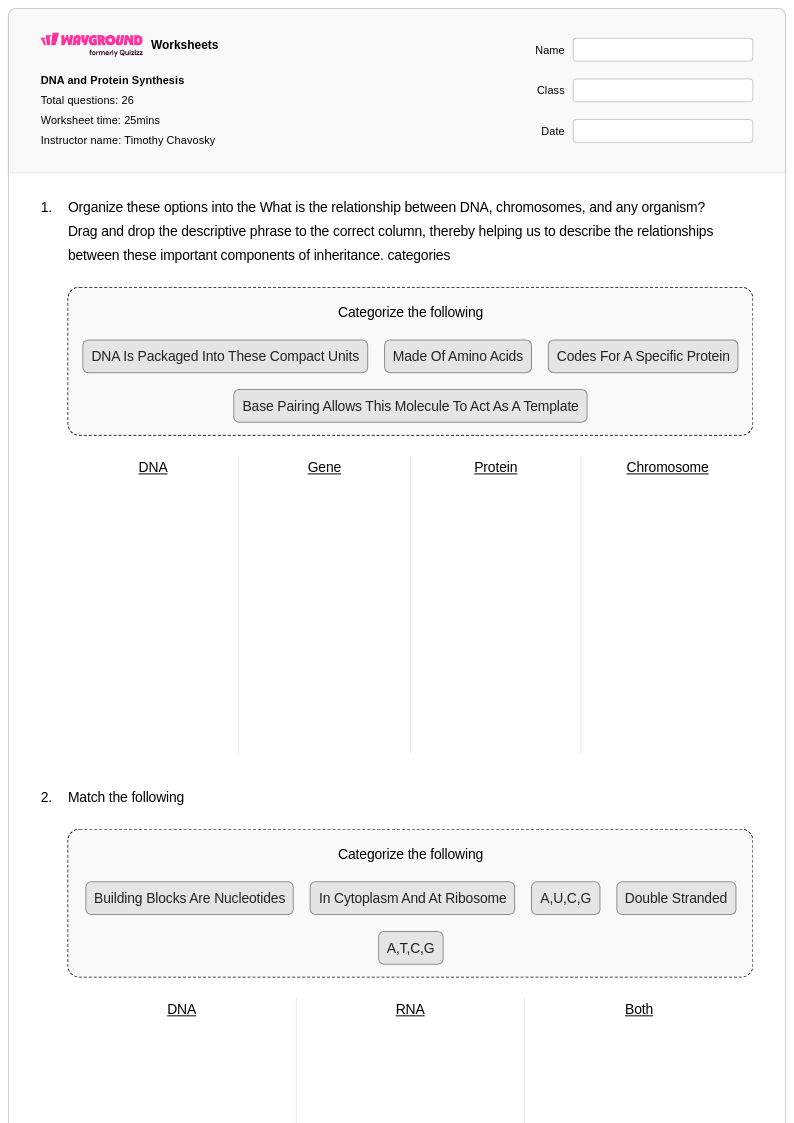
26Q
9th - 12th
Explore otras hojas de trabajo de materias para class 12
Explore printable Conjugate Acid Base worksheets for Class 12
Conjugate acid base pairs represent one of the most fundamental concepts in Class 12 chemistry, requiring students to master the intricate relationships between acids, bases, and their corresponding conjugate species according to Brønsted-Lowry theory. Wayground's comprehensive collection of conjugate acid base worksheets provides students with systematic practice problems that develop critical skills in identifying conjugate pairs, predicting relative strengths, and analyzing proton transfer reactions in aqueous solutions. These carefully crafted printables feature progressive difficulty levels that guide students from basic identification exercises to complex equilibrium calculations involving Ka and Kb relationships, with each worksheet including a detailed answer key that explains the reasoning behind conjugate pair formations and strength comparisons. The free pdf resources emphasize conceptual understanding while building computational proficiency in calculating pH, pOH, and equilibrium concentrations for conjugate acid base systems.
Wayground's extensive library of teacher-created conjugate acid base worksheets draws from millions of educational resources developed by chemistry educators who understand the complexities of this advanced topic. The platform's sophisticated search and filtering capabilities allow teachers to locate materials that align with specific curriculum standards while offering differentiation tools to accommodate varying student ability levels within Class 12 chemistry courses. Teachers can customize these printable and digital pdf worksheets to focus on particular aspects of conjugate acid base theory, whether for initial instruction, targeted remediation of misconceptions about relative strengths, or enrichment activities involving polyprotic acids and complex equilibrium systems. This flexibility supports comprehensive lesson planning by providing ready-to-use materials that can be seamlessly integrated into laboratory work, homework assignments, and assessment preparation, ensuring students develop a thorough understanding of how conjugate pairs govern acid base behavior in chemical systems.
