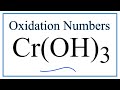
Oxidation States and Numbers in Chemistry
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of chromium(III) hydroxide?
Positive
Negative
Neutral
Depends on the environment
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the first method, what is the charge of the hydroxide ion?
-2
+1
-1
0
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydroxide ions are present in chromium(III) hydroxide?
1
4
3
2
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of chromium in chromium(III) hydroxide using the first method?
+1
+2
+3
+4
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the second method, what is the assumed oxidation state of oxygen?
-1
-2
+1
0
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation state of hydrogen when bonded to oxygen?
+1
+2
-1
0
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What equation is set up to find the oxidation number of chromium?
x + 3 = 0
x - 3 = 0
x + 3 = 3
x - 3 = 3
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of chromium in chromium(III) hydroxide using the second method?
+3
+1
+2
+4
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Do both methods yield the same oxidation number for chromium?
No
Yes
Depends on the method used
Only in specific cases
Similar Resources on Wayground

8 questions
Oxidation Numbers in Compounds
Interactive video
•
9th - 10th Grade

7 questions
Oxidation Numbers in Compounds
Interactive video
•
9th - 10th Grade

8 questions
Understanding Chromium and Nitrite Ions
Interactive video
•
9th - 10th Grade

9 questions
Chromium Hydroxide and Ion Charges
Interactive video
•
9th - 10th Grade

6 questions
Understanding Chromium Ions and Oxidation Numbers
Interactive video
•
9th - 10th Grade

8 questions
Oxidation Numbers and Chromium Compounds
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Hydroxides and Ions
Interactive video
•
9th - 10th Grade

9 questions
Oxidation Numbers and Iron Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade
Discover more resources for Chemistry

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

24 questions
Scientific method and variables review
Quiz
•
9th Grade

10 questions
Characteristics of Life
Quiz
•
9th - 10th Grade

19 questions
Mental Health Vocabulary Pre-test
Quiz
•
9th Grade

14 questions
Points, Lines, Planes
Quiz
•
9th Grade