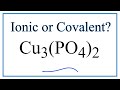
Copper(II) Phosphate Charge and Bonding
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is Copper(II) Phosphate primarily considered?
Metallic
Polar
Covalent
Ionic
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which elements in Copper(II) Phosphate are non-metals?
Copper and Hydrogen
Copper and Oxygen
Phosphorus and Oxygen
Copper and Phosphorus
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of a single phosphate ion in Copper(II) Phosphate?
2-
3+
2+
3-
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many copper ions are needed to balance the charge of two phosphate ions?
Four
Three
Two
One
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the overall charge of Copper(II) Phosphate?
Positive
Negative
Variable
Neutral
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of each copper ion in Copper(II) Phosphate?
3+
4+
1+
2+
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bond is formed between phosphorus and oxygen in the phosphate ion?
Metallic
Hydrogen
Covalent
Ionic
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Magnesium Phosphate Bonding and Properties
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Copper II Phosphate
Interactive video
•
9th - 10th Grade

10 questions
Copper II Phosphate Molar Mass Concepts
Interactive video
•
9th - 10th Grade

11 questions
Chemical Reactions and Ionic Equations
Interactive video
•
9th - 10th Grade

8 questions
Phosphate Compounds and Ions
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

8 questions
Solubility and Properties of Phosphates
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Reactions and Phosphate Groups
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

10 questions
Chaffey
Quiz
•
9th - 12th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

22 questions
6-8 Digital Citizenship Review
Quiz
•
6th - 8th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab safety
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

12 questions
Counting Significant Figures Quick Check
Quiz
•
9th - 12th Grade

10 questions
Significant Figures Int 2
Quiz
•
9th - 12th Grade

19 questions
States of Matter Review
Quiz
•
10th Grade

21 questions
Lab Safety
Quiz
•
10th Grade