Acids and Bases (AP Chem)
Authored by Caroline Duesing
Chemistry
11th - 12th Grade
NGSS covered
Used 326+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

A buffer solution is prepared by mixing equal volumes of 0.50 M weak acid with 1.0 M of its conjugate base. Based on the data given in the table above, which of the following pairs of chemical solutions should be used to prepare the buffer solution so that the pH will be between 4 and 7?
CH3COOH and NH3
CH3COOH and CH3COONa
H2CO3 and NH3
H2CO3 and Na2CO3
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Ascorbic acid, H2C6H6O6(s), is a diprotic acid with K1 = 7.9 × 10–5 and K2 = 1.6 × 10–12. In a 0.005 M aqueous solution of ascorbic acid, which of the following species is present in the lowest concentration?
H3O+(aq)
H2C6H6O6(aq)
HC6H6O6-(aq)
C6H6O62-(aq)
Tags
NGSS.HS-PS1-7
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
In the titration of a weak acid of unknown concentration with a standard solution of a strong base, a pH meter was used to follow the progress of the titration. Which of the following is true for this experiment?
The [H+] at the equivalence point equals the ionization constant of the acid.
The pH at the equivalence point depends on the indicator used.
The graph of pH versus volume of base added rises gradually at first and then much more rapidly.
The graph of pH versus volume of base added shows no sharp rise.
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following is the correct equilibrium expression for the hydrolysis of CO3 2– ?
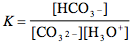
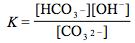
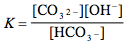
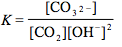
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Which of the following best approximates the Ka value for this weak acid?
1 x 10–3
1 x 10–4
1 x 10–5
1 x 10–6
6.
MULTIPLE CHOICE QUESTION
45 sec • 1 pt

Which point on the titration curve corresponds to the point at which the moles of the added strong base are equal to the moles of the weak acid initially present?
Q
R
S
T
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

At point P in the titration, which of the following species has the highest concentration?
HA
A–
H3O+
OH–
Tags
NGSS.HS-PS1-3
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade