
10 Q
12th

18 Q
9th

18 Q
10th

25 Q
9th - Uni

15 Q
10th - Uni

25 Q
10th - Uni

14 Q
11th

40 Q
9th - 12th

15 Q
10th - Uni
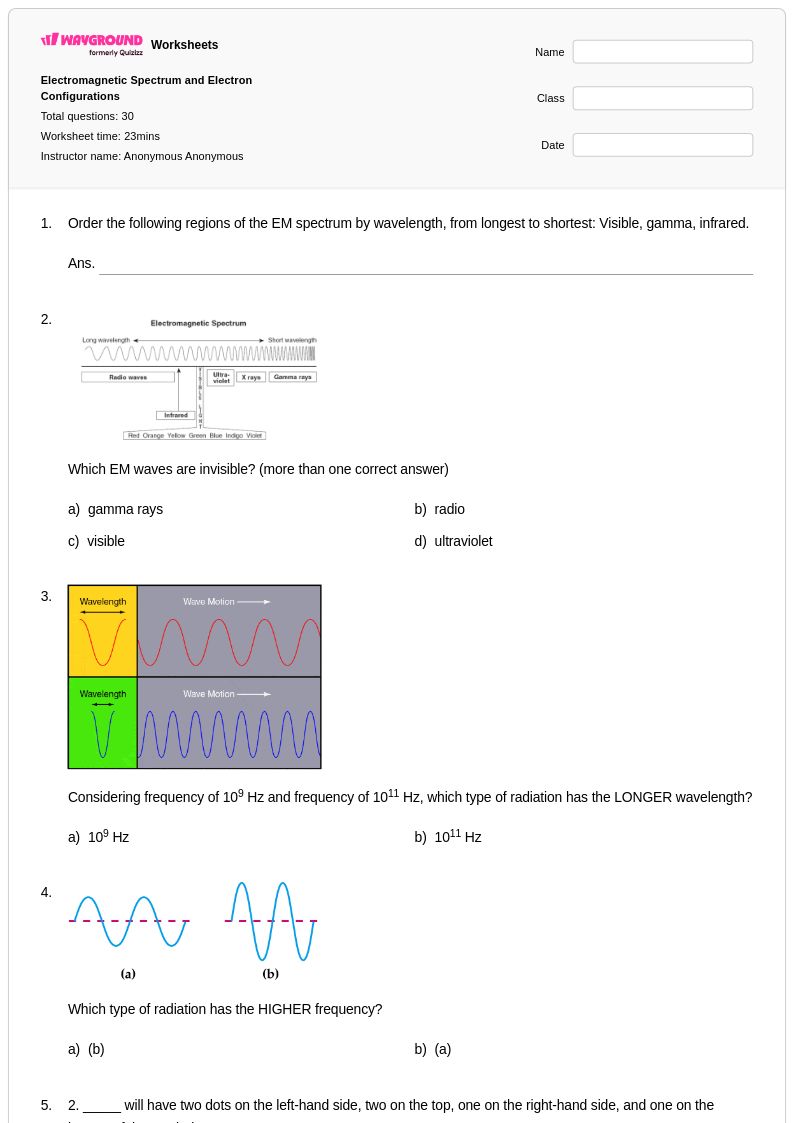
30 Q
10th

20 Q
10th

20 Q
10th
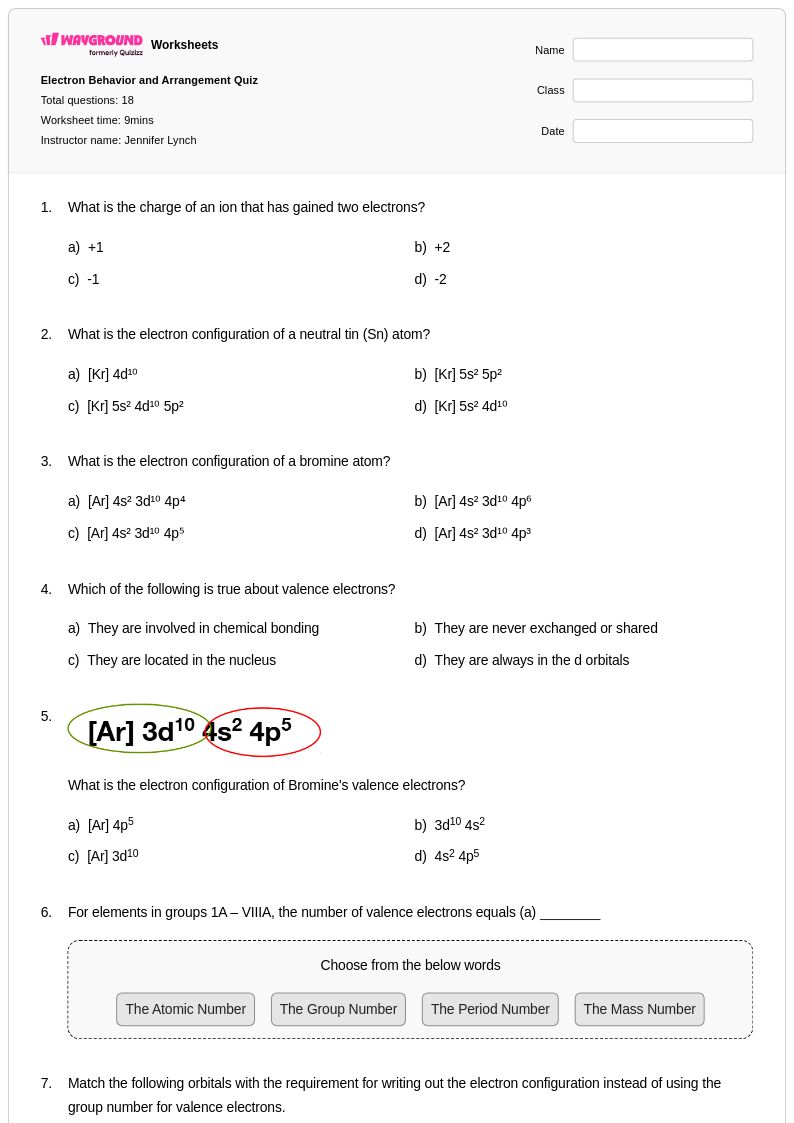
18 Q
11th - Uni
25 Q
10th - Uni

25 Q
10th - Uni
25 Q
11th - Uni
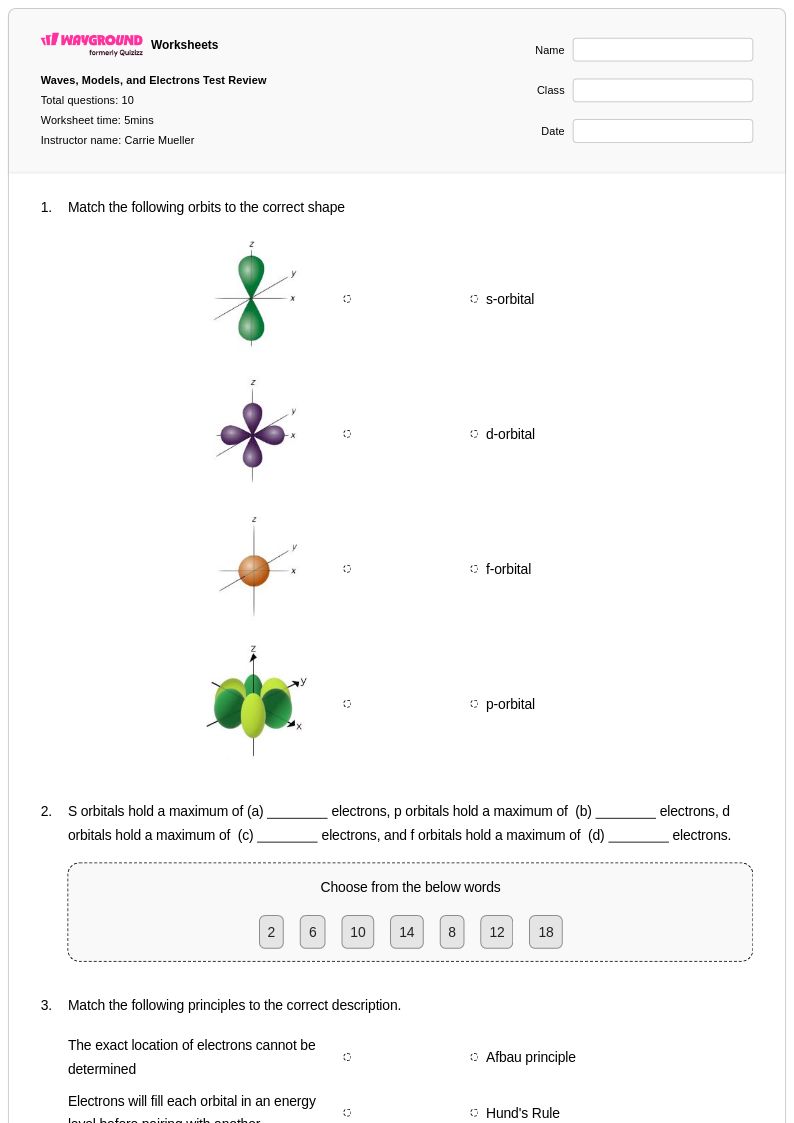
10 Q
10th
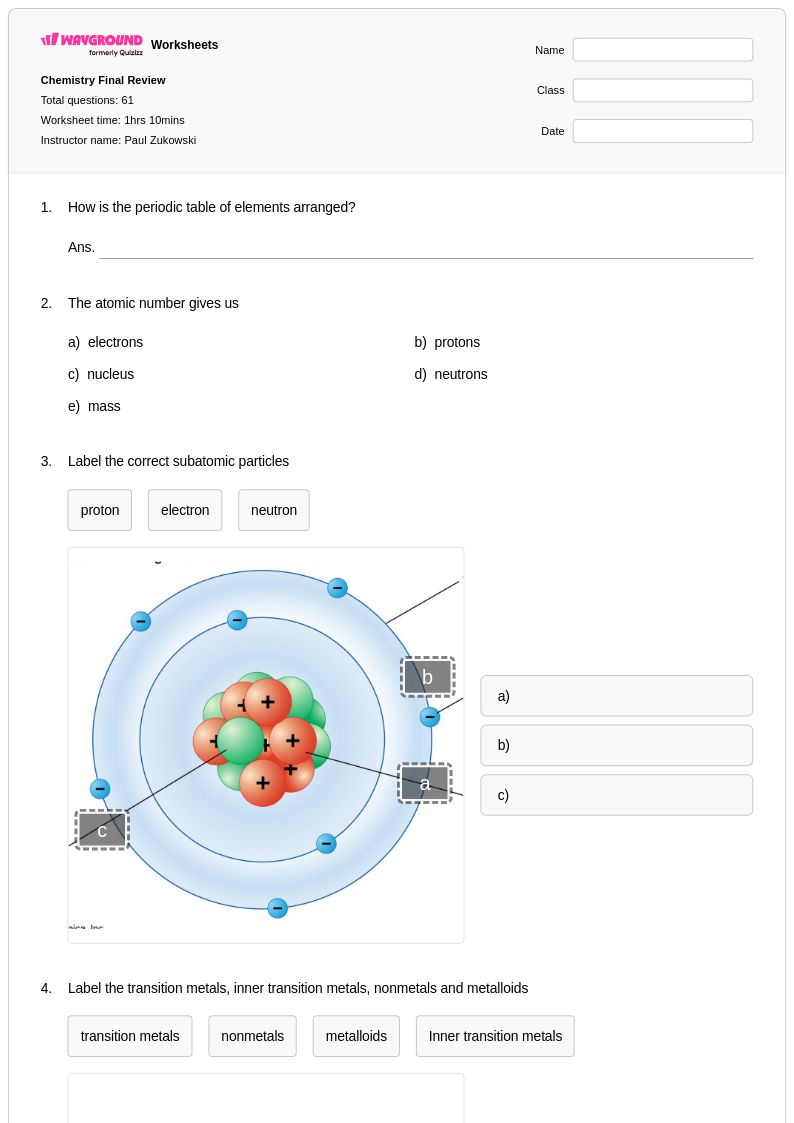
61 Q
12th
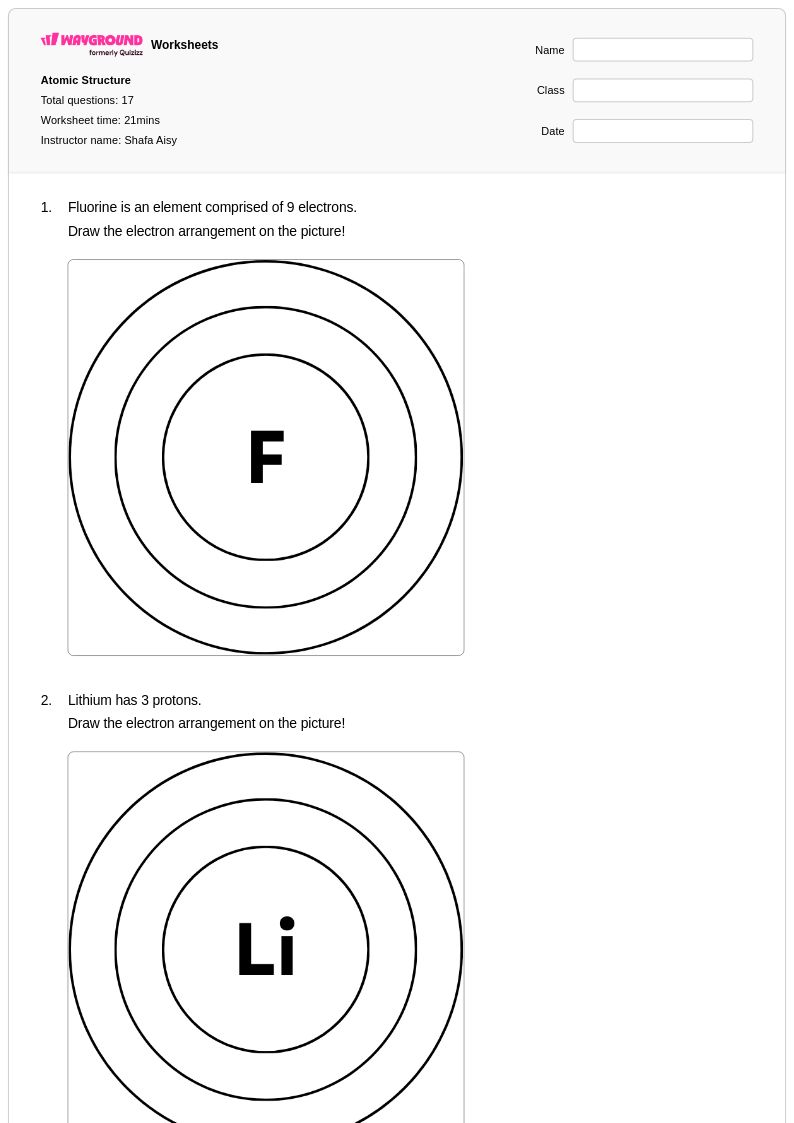
17 Q
9th

12 Q
8th

18 Q
11th

15 Q
10th

20 Q
8th

25 Q
10th
Explore Worksheets by Subjects
Explore printable Electron Configuration worksheets
Electron configuration worksheets available through Wayground (formerly Quizizz) provide comprehensive practice for students learning to determine how electrons are distributed in atoms and ions. These expertly designed resources strengthen fundamental chemistry skills including writing electron configurations using orbital notation, noble gas notation, and electron dot diagrams, while helping students understand periodic trends and the relationship between electron arrangement and chemical properties. The extensive collection includes practice problems ranging from basic ground state configurations to more advanced concepts like excited states and ionization, with each worksheet featuring detailed answer keys and available as free printable pdf downloads that support both classroom instruction and independent study.
Wayground (formerly Quizizz) empowers chemistry teachers with millions of teacher-created electron configuration resources that feature robust search and filtering capabilities to quickly locate materials aligned with specific learning standards and student needs. The platform's differentiation tools allow educators to customize worksheets for various skill levels, from introductory atomic structure concepts to advanced quantum mechanical principles, while offering both printable pdf formats for traditional assignments and digital versions for interactive learning experiences. These flexible resources support comprehensive lesson planning by providing teachers with ready-made materials for initial instruction, targeted remediation for struggling students, enrichment activities for advanced learners, and ongoing skill practice that reinforces the critical connection between electron configuration and chemical behavior throughout the periodic table.
