18 P
10th
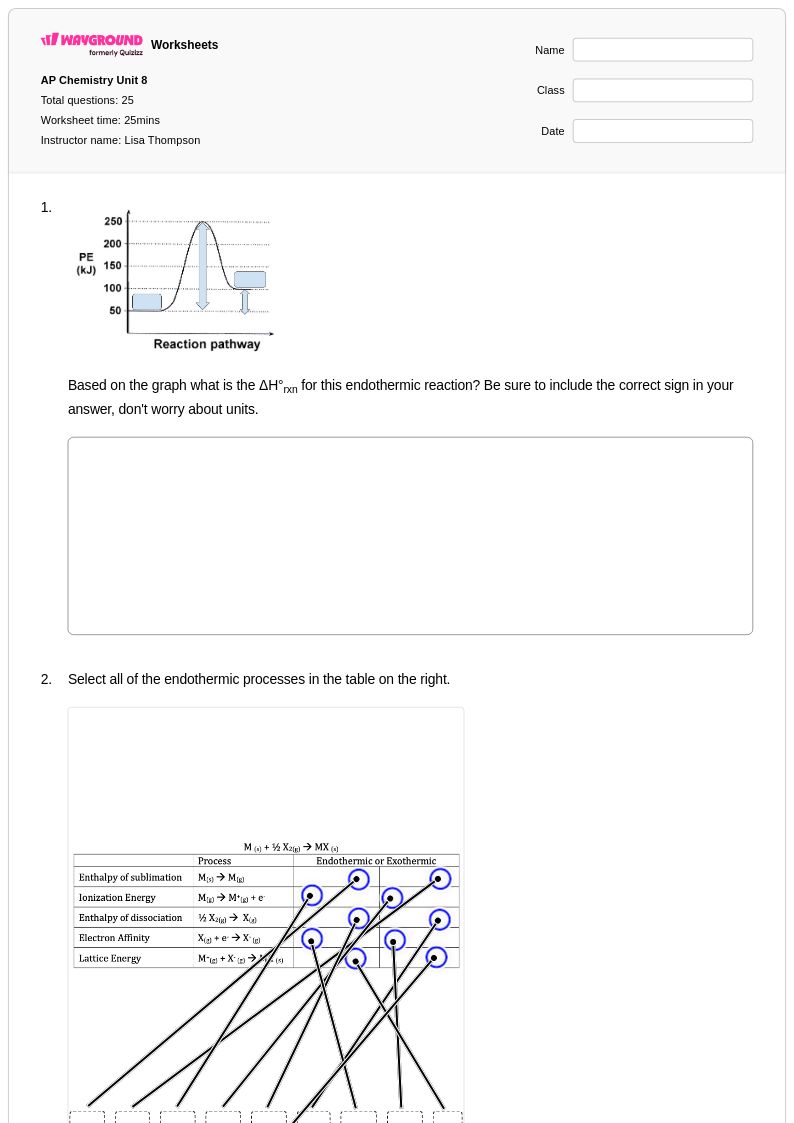
25 P
11th - Uni

25 P
7th - Uni
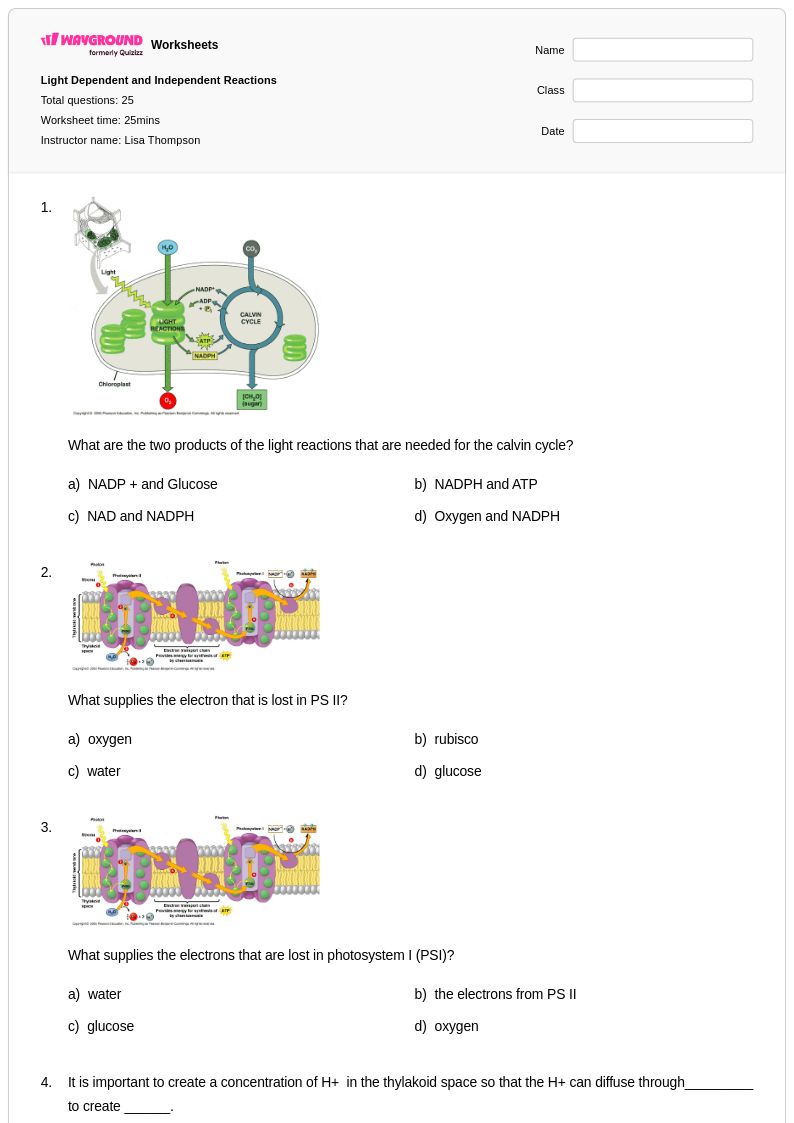
25 P
9th - Uni

10 P
10th
12 P
9th - Uni
15 P
10th - Uni
15 P
10th - Uni

25 P
8th - Uni

19 P
9th
25 P
9th - Uni
18 P
8th

20 P
8th
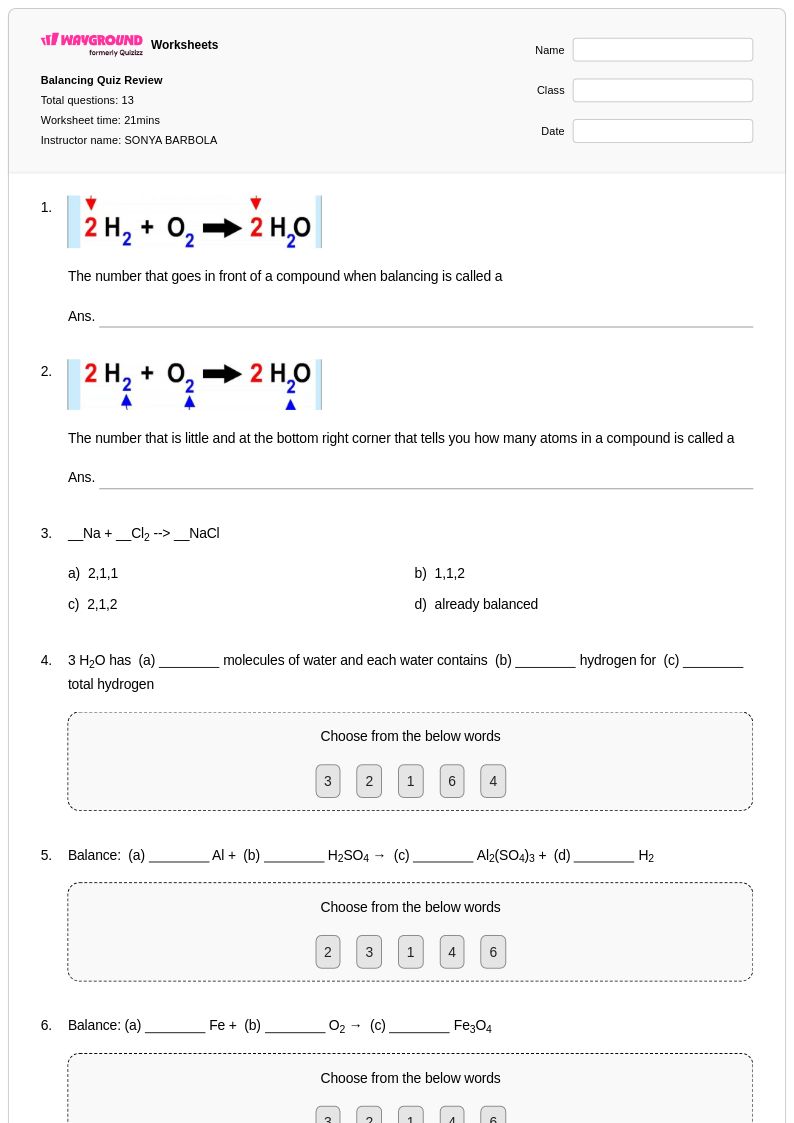
13 P
9th - 12th

15 P
9th - Uni

15 P
11th - Uni
75 P
9th

25 P
10th - Uni
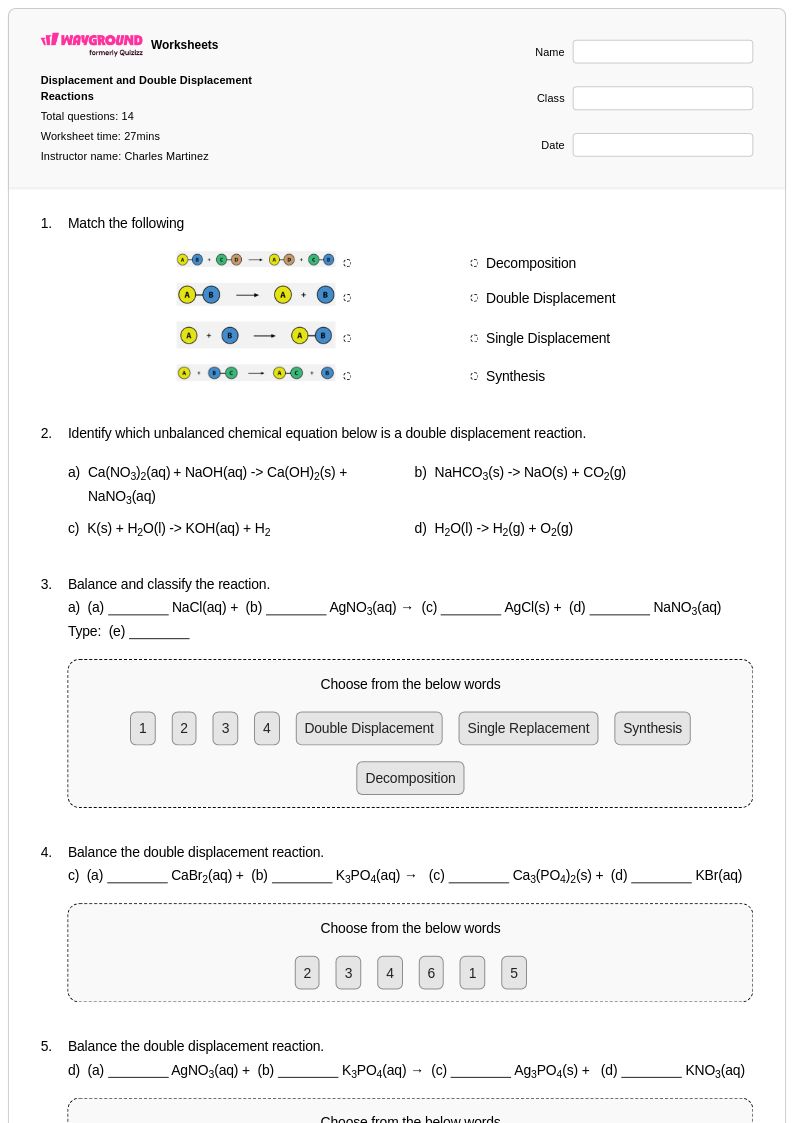
14 P
10th
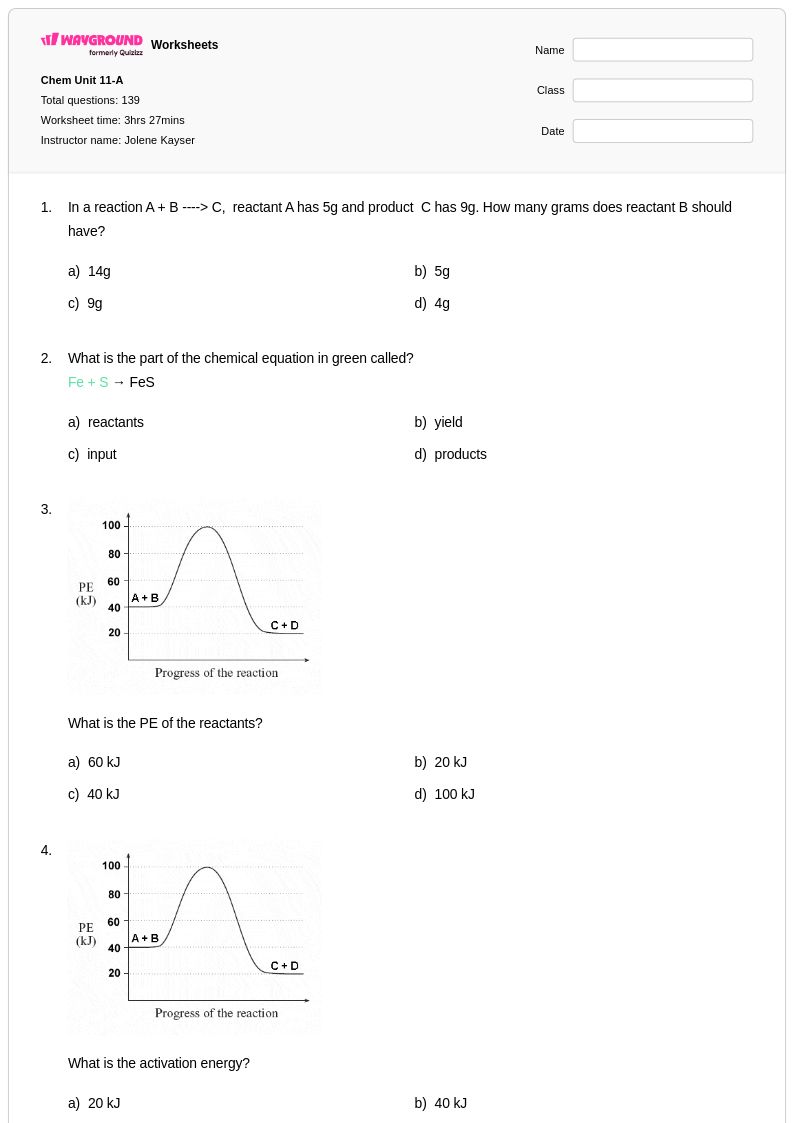
139 P
9th - 12th

15 P
9th - Uni
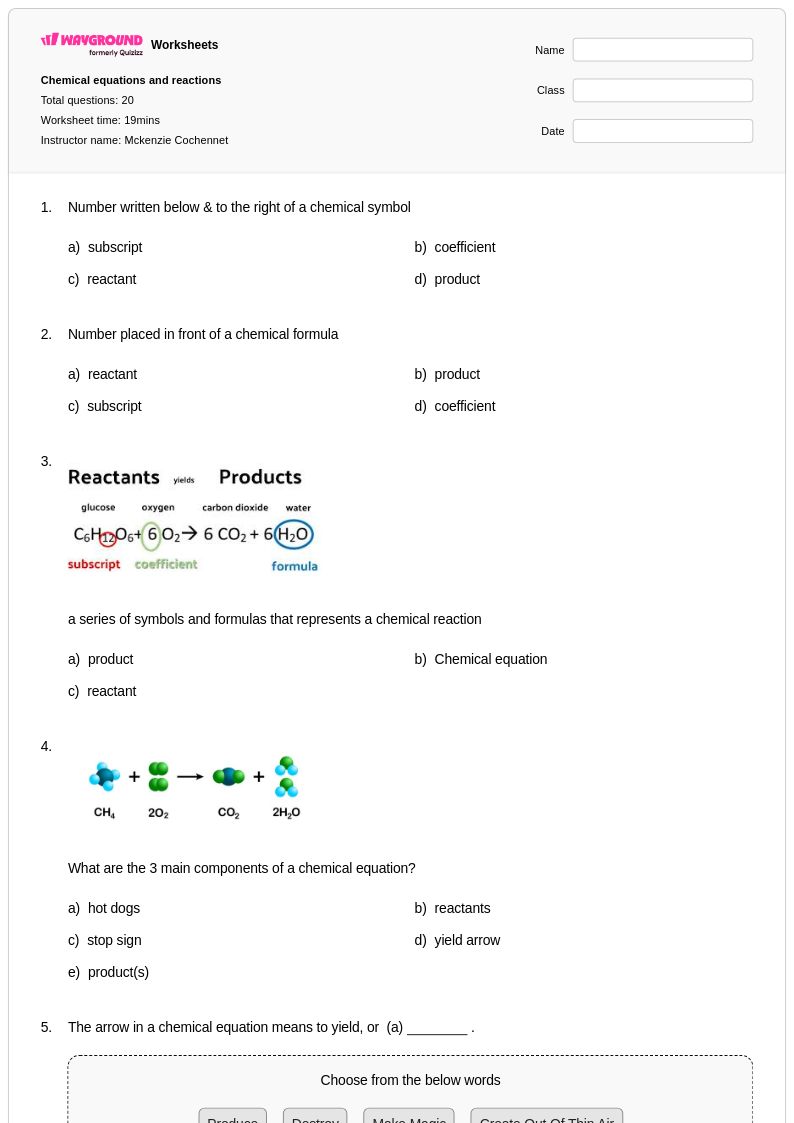
20 P
8th

17 P
6th
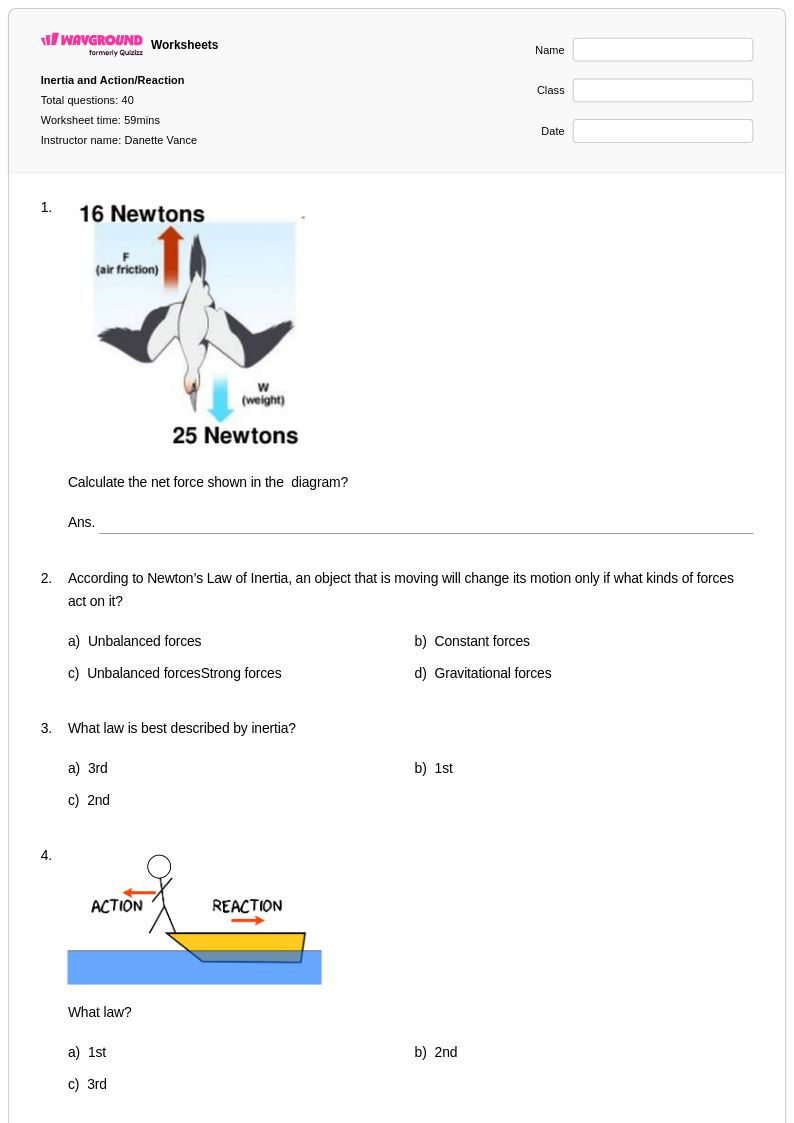
40 P
8th
Przeglądaj arkusze według tematów
Explore printable Equilibrium Constant and Reaction Quotient worksheets
Equilibrium constant and reaction quotient worksheets available through Wayground provide comprehensive practice with these fundamental chemical equilibrium concepts that form the backbone of advanced chemistry studies. These expertly designed resources help students master the calculation and interpretation of equilibrium constants (Kc, Kp, and Ksp), understand the relationship between reaction quotients and equilibrium positions, and develop proficiency in predicting reaction direction and extent. The worksheet collections include detailed practice problems that progress from basic equilibrium constant calculations to complex scenarios involving multiple equilibria and Le Chatelier's principle applications. Each resource comes with comprehensive answer keys and is available as free printable pdf downloads, making them accessible for both classroom instruction and independent study sessions.
Wayground's extensive library of millions of teacher-created equilibrium constant and reaction quotient worksheets provides educators with unparalleled flexibility in delivering this challenging chemistry content. The platform's robust search and filtering capabilities allow teachers to quickly locate materials that align with specific curriculum standards and match their students' varying skill levels, from introductory equilibrium concepts to advanced thermodynamic applications. These differentiation tools enable seamless customization of worksheets for remediation support, enrichment activities, and targeted skill practice, while the availability of both digital and printable pdf formats ensures compatibility with diverse classroom environments and teaching styles. Teachers can efficiently plan comprehensive lesson sequences that build students' confidence with equilibrium calculations while providing the repetitive practice necessary for mastering these mathematically intensive chemistry concepts.
