18 คิว
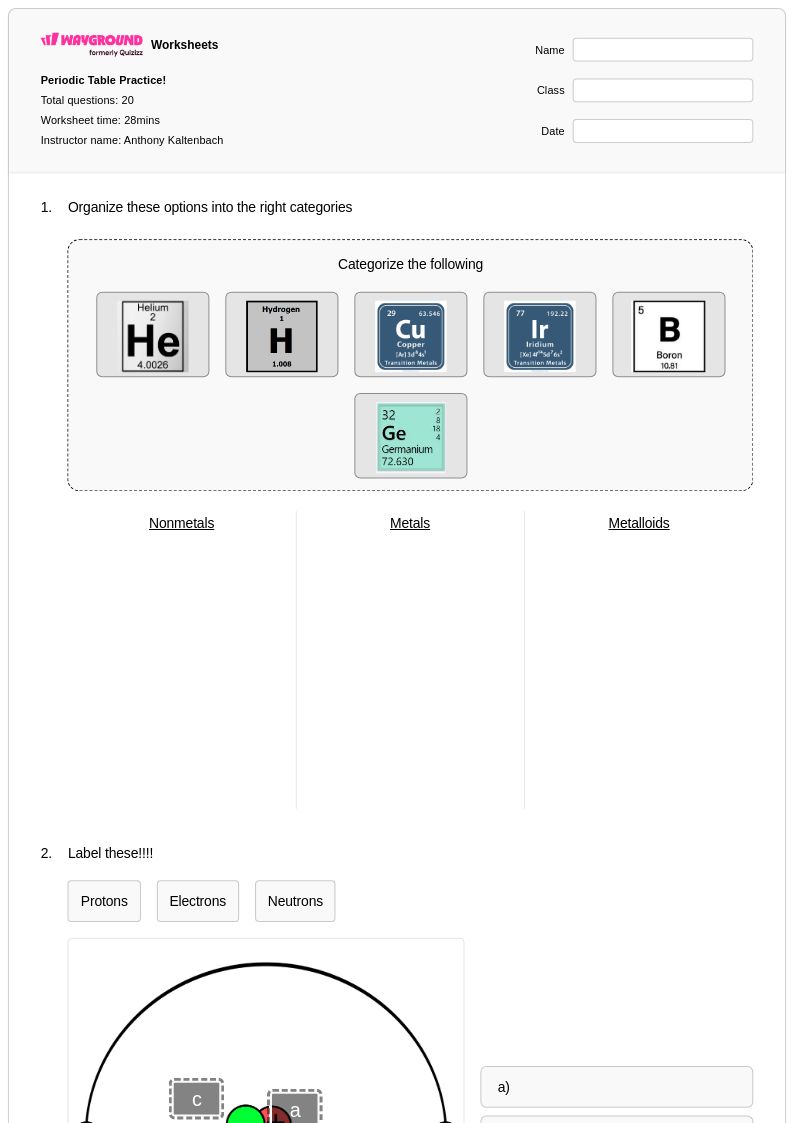
11th

20 คิว
7th
16 คิว
6th

15 คิว
6th

11 คิว
KG

17 คิว
7th

26 คิว
7th

13 คิว
6th
20 คิว
6th

14 คิว
10th

26 คิว
8th
25 คิว
9th - 12th

18 คิว
6th

14 คิว
7th - Uni

14 คิว
Uni
20 คิว
7th

15 คิว
12th - Uni
25 คิว
10th - Uni

23 คิว
10th

20 คิว
10th

13 คิว
9th - 12th

17 คิว
9th

22 คิว
7th
สำรวจแผ่นงานตามหัวเรื่อง
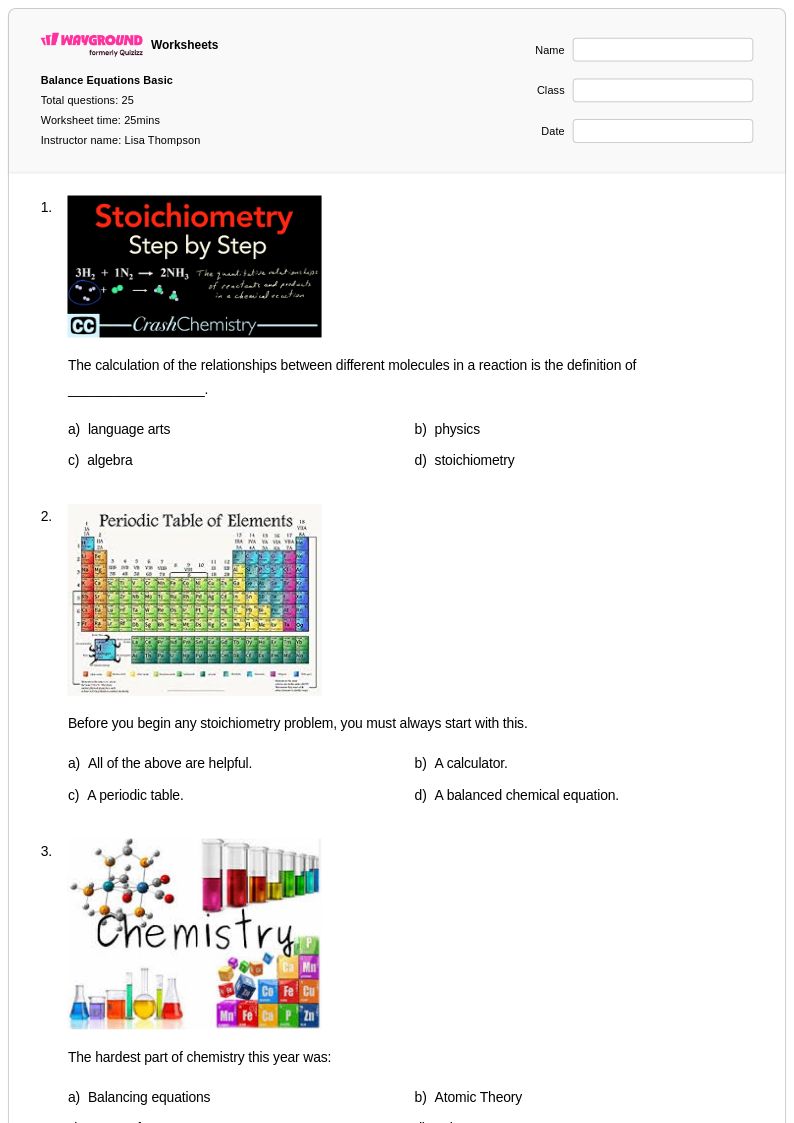
Explore printable Stoichiometry of Gases worksheets
Stoichiometry of gases worksheets available through Wayground (formerly Quizizz) provide comprehensive practice materials that strengthen students' ability to apply quantitative relationships to gaseous reactants and products in chemical reactions. These expertly crafted resources focus on essential skills including mole-to-mole conversions involving gases, calculating volumes at standard temperature and pressure, applying the ideal gas law to stoichiometric problems, and determining limiting reactants when gases are involved in chemical processes. The collection includes detailed practice problems that guide students through complex multi-step calculations, complete answer keys for immediate feedback and self-assessment, and free printables that reinforce conceptual understanding of how the molar volume of gases relates to balanced chemical equations.
Wayground (formerly Quizizz) empowers chemistry educators with millions of teacher-created stoichiometry of gases resources that streamline lesson planning and support diverse learning needs in the classroom. The platform's robust search and filtering capabilities allow teachers to quickly locate materials aligned with specific chemistry standards and learning objectives, while built-in differentiation tools enable customization of problem complexity to match individual student readiness levels. These comprehensive worksheet collections are available in both printable pdf formats for traditional paper-based practice and interactive digital versions that provide instant feedback, making them invaluable for targeted skill practice, remediation of challenging gas law concepts, and enrichment activities that challenge advanced learners to master sophisticated stoichiometric calculations involving gaseous systems.
