
18 Q
9th - 12th
15 Q
8th - Uni

20 Q
11th - 12th
15 Q
12th - Uni
14 Q
9th

19 Q
9th - 11th

15 Q
9th

55 Q
8th
46 Q
8th

24 Q
7th
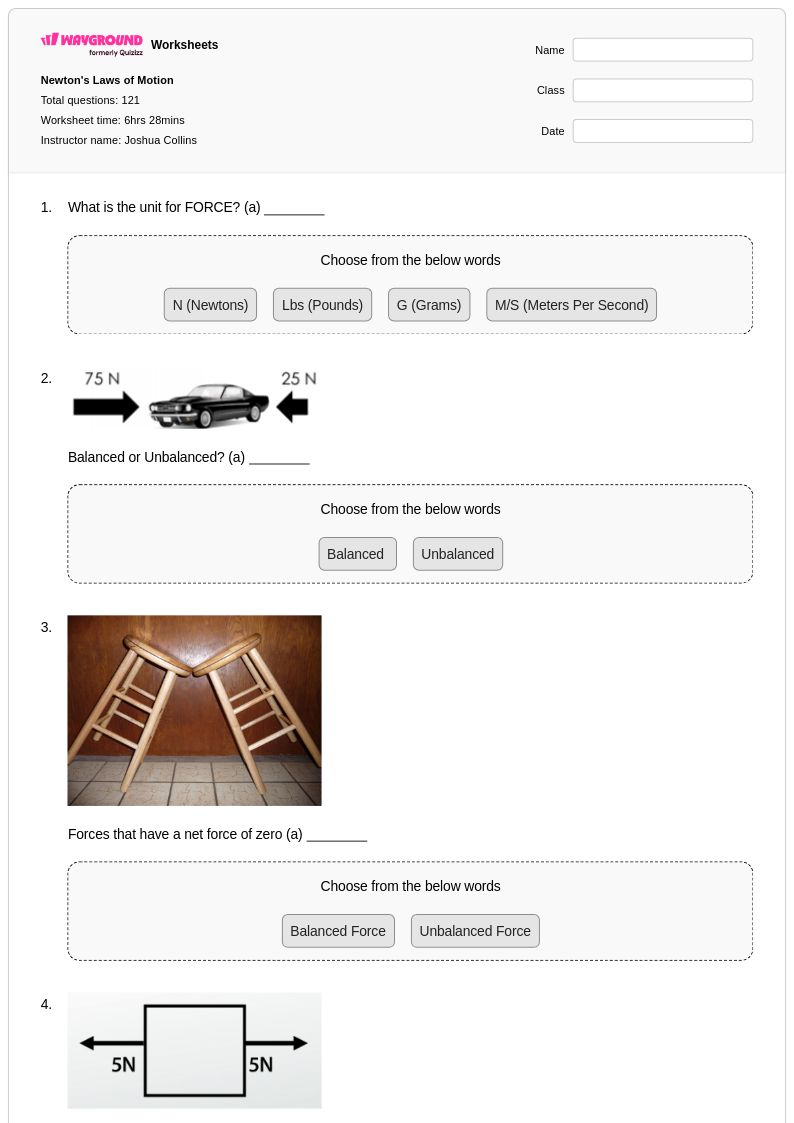
121 Q
8th
21 Q
6th
10 Q
8th

35 Q
8th
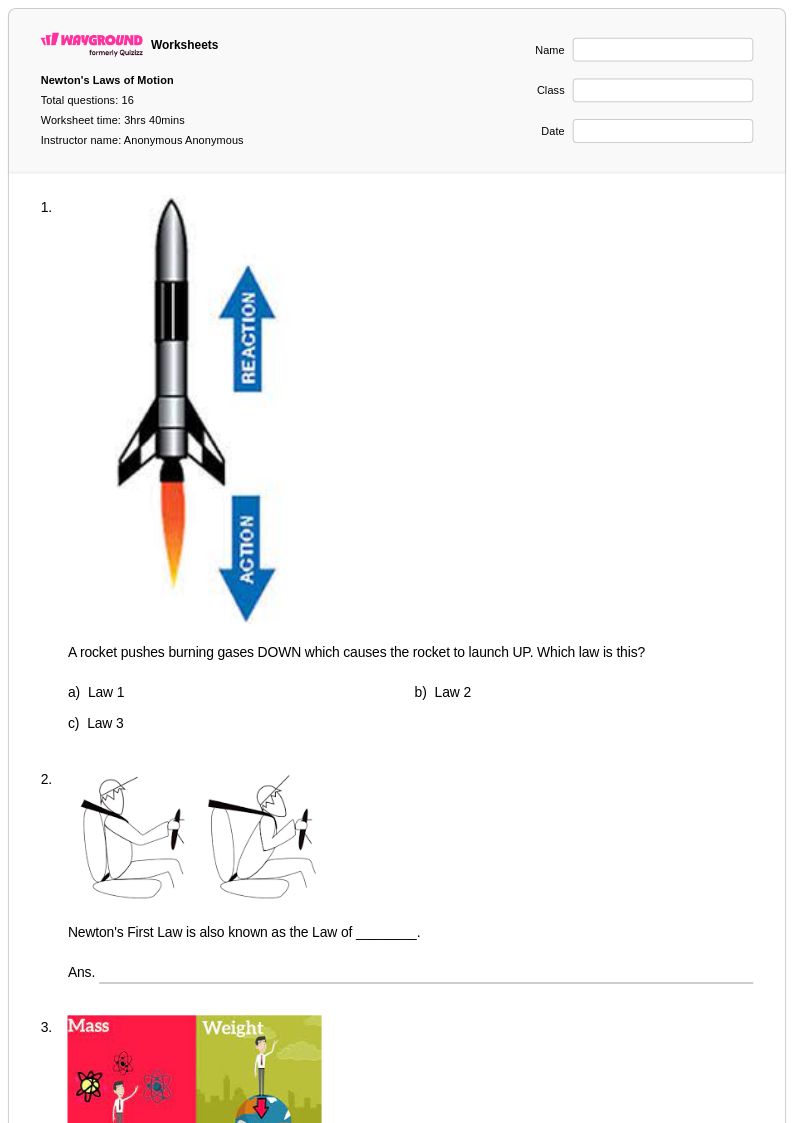
16 Q
8th
20 Q
10th
15 Q
8th
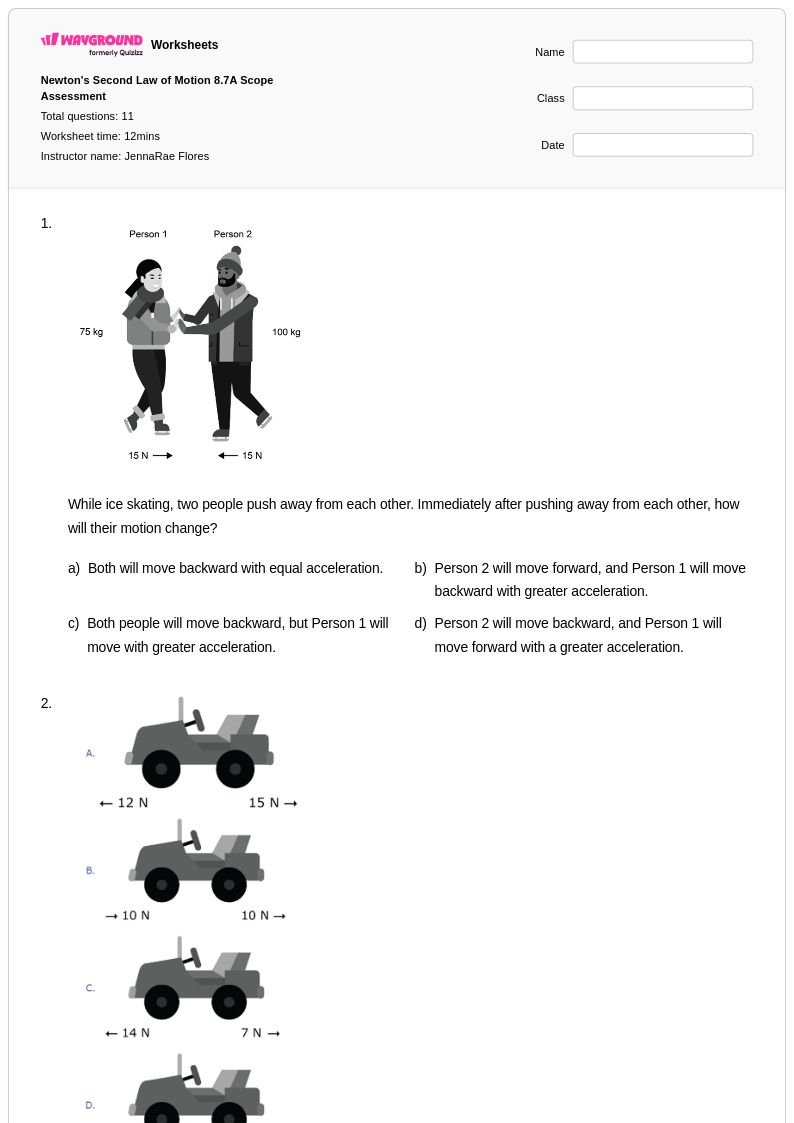
11 Q
8th
67 Q
8th

13 Q
7th

30 Q
8th

19 Q
8th

21 Q
4th - 6th
Explore Worksheets by Subjects
Explore printable Rate Laws worksheets
Rate laws worksheets available through Wayground (formerly Quizizz) provide comprehensive practice opportunities for students to master the fundamental principles governing chemical reaction rates. These carefully designed educational resources focus on helping learners understand how to determine reaction orders, calculate rate constants, and interpret experimental data to derive rate law expressions. Students develop critical analytical skills as they work through practice problems involving integrated rate laws, half-life calculations, and the relationship between concentration and time in zero, first, and second-order reactions. The worksheets include detailed answer keys that guide students through complex problem-solving strategies, and many are available as free printables in convenient pdf format, making them accessible for both classroom instruction and independent study.
Wayground (formerly Quizizz) empowers educators with millions of teacher-created rate laws resources that streamline lesson planning and enhance student learning outcomes. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific chemistry standards and curriculum requirements, whether focusing on elementary rate law concepts or advanced kinetics applications. These versatile materials support differentiated instruction through customizable difficulty levels and can be seamlessly adapted for various learning environments in both printable and digital formats. Teachers utilize these comprehensive worksheet collections for targeted skill practice, remediation of challenging kinetics concepts, and enrichment activities that deepen student understanding of reaction mechanisms and catalysis, ultimately building a stronger foundation in chemical kinetics across diverse academic settings.
