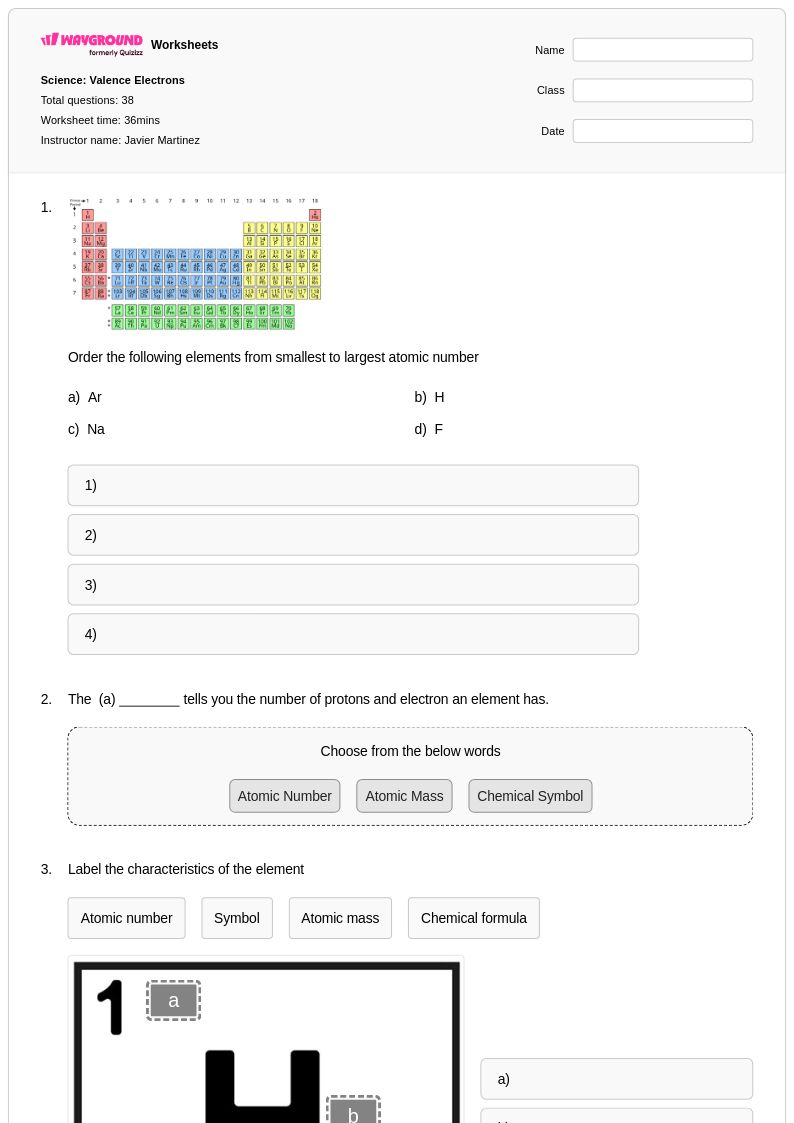
38 Q
7th
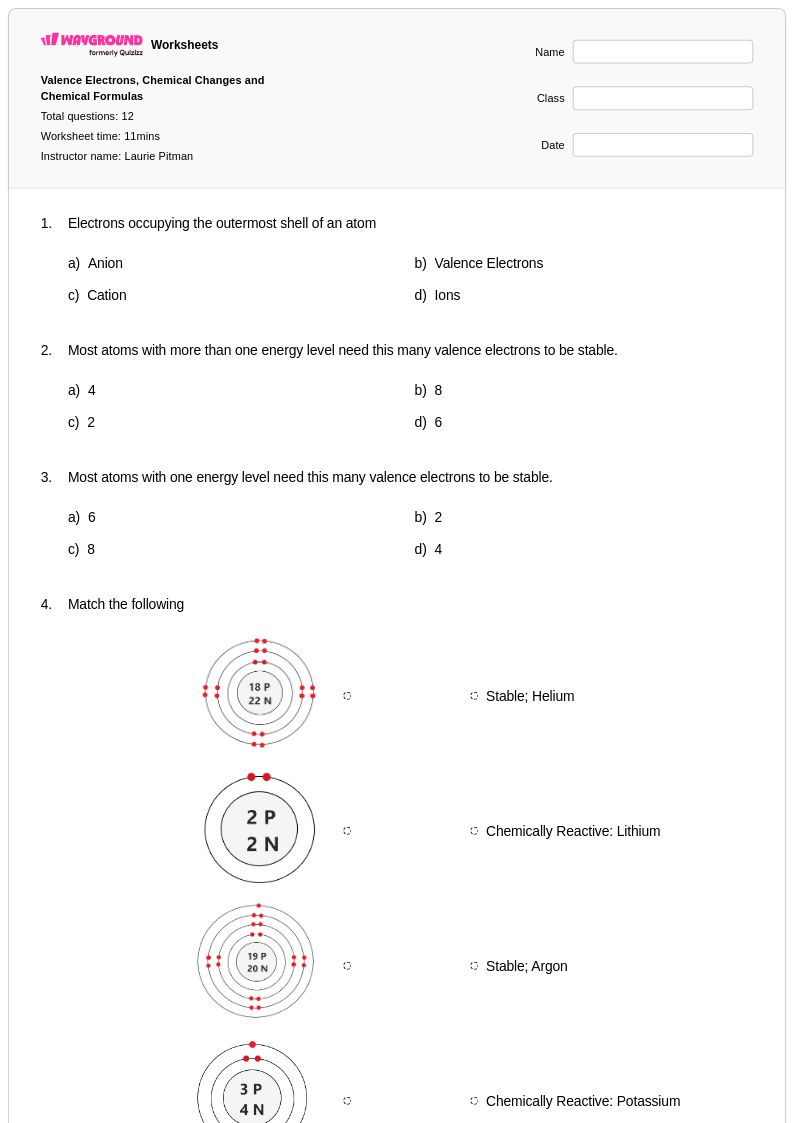
12 Q
6th - 8th
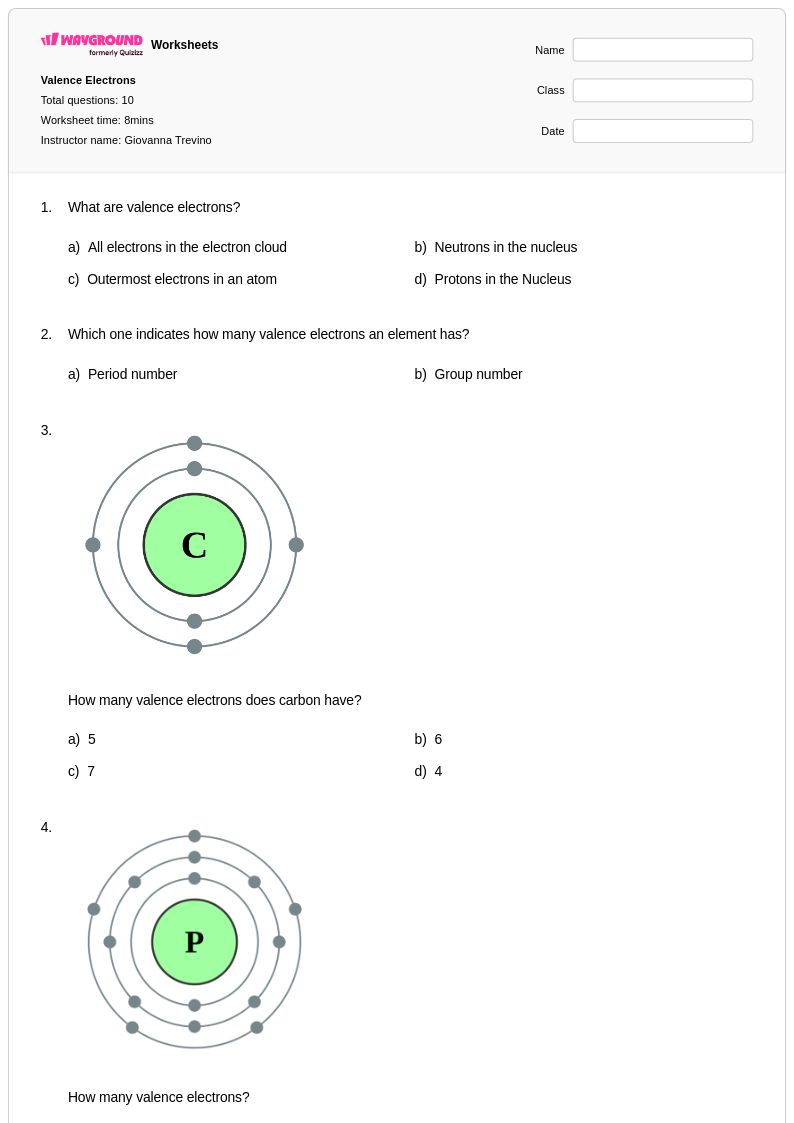
10 Q
7th

15 Q
7th

15 Q
7th

60 Q
6th - 8th
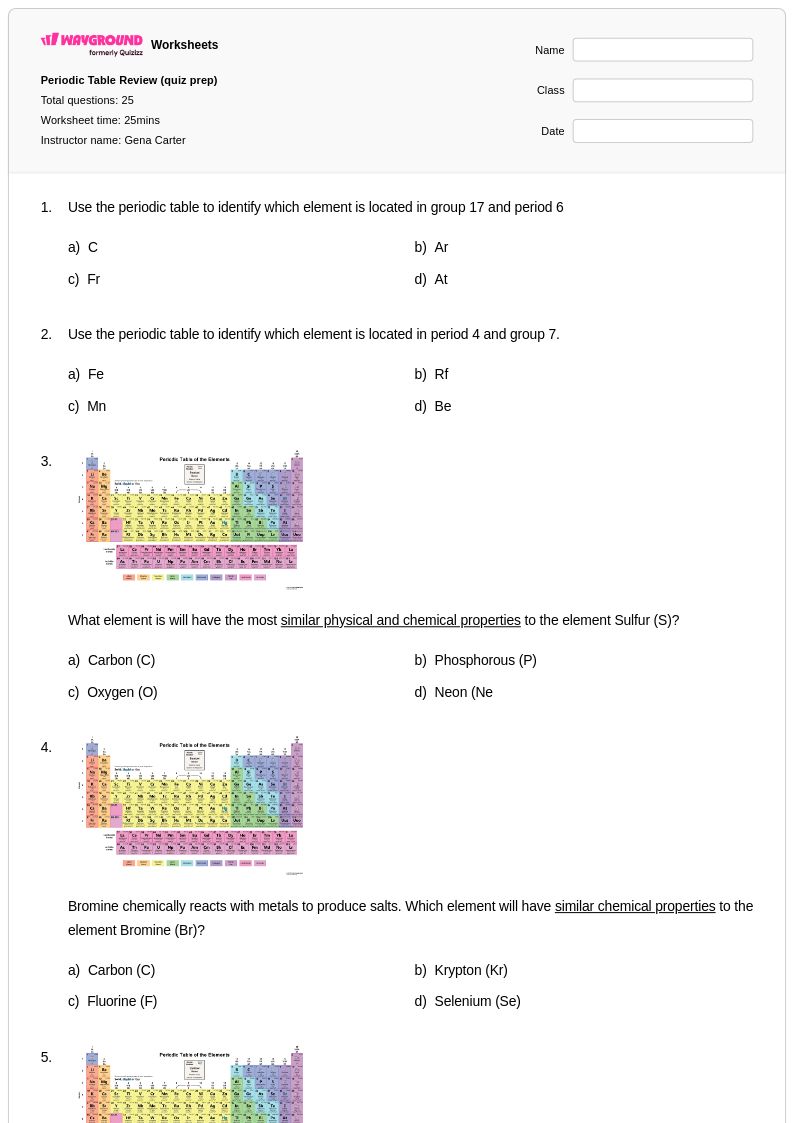
25 Q
7th
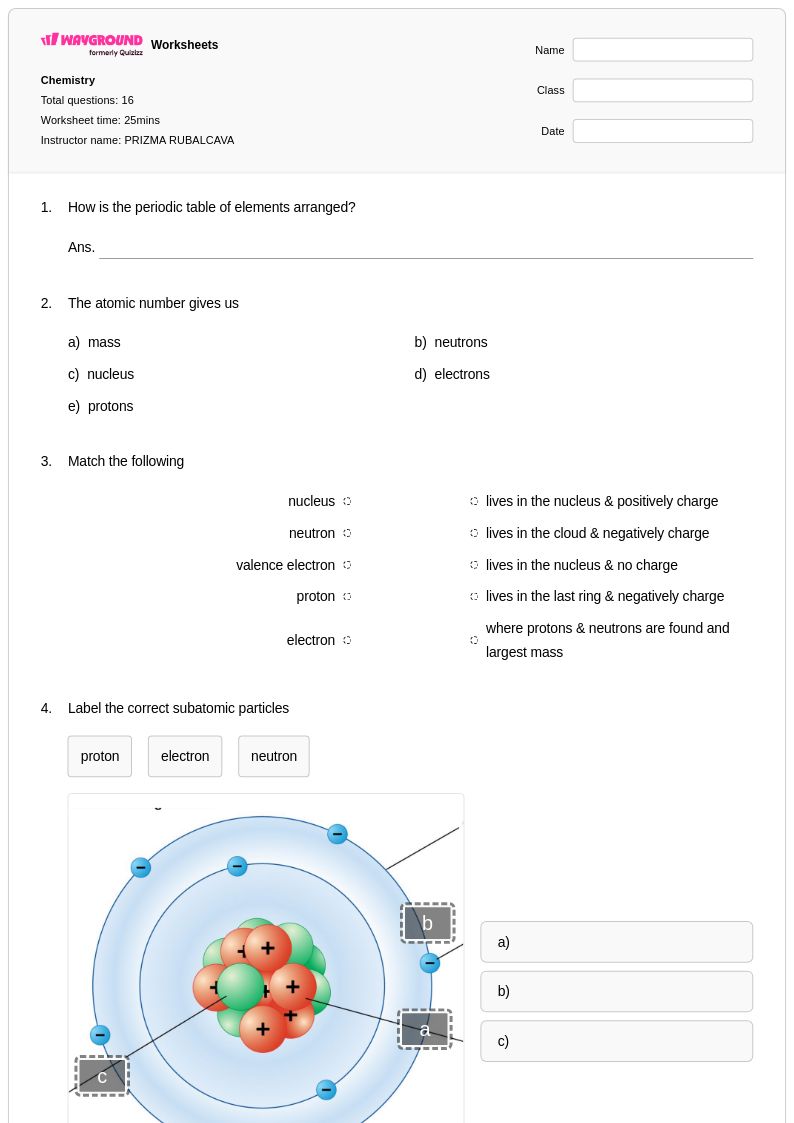
16 Q
6th - 8th
18 Q
7th
25 Q
6th - 8th
18 Q
7th

66 Q
7th

19 Q
7th

37 Q
6th - 8th
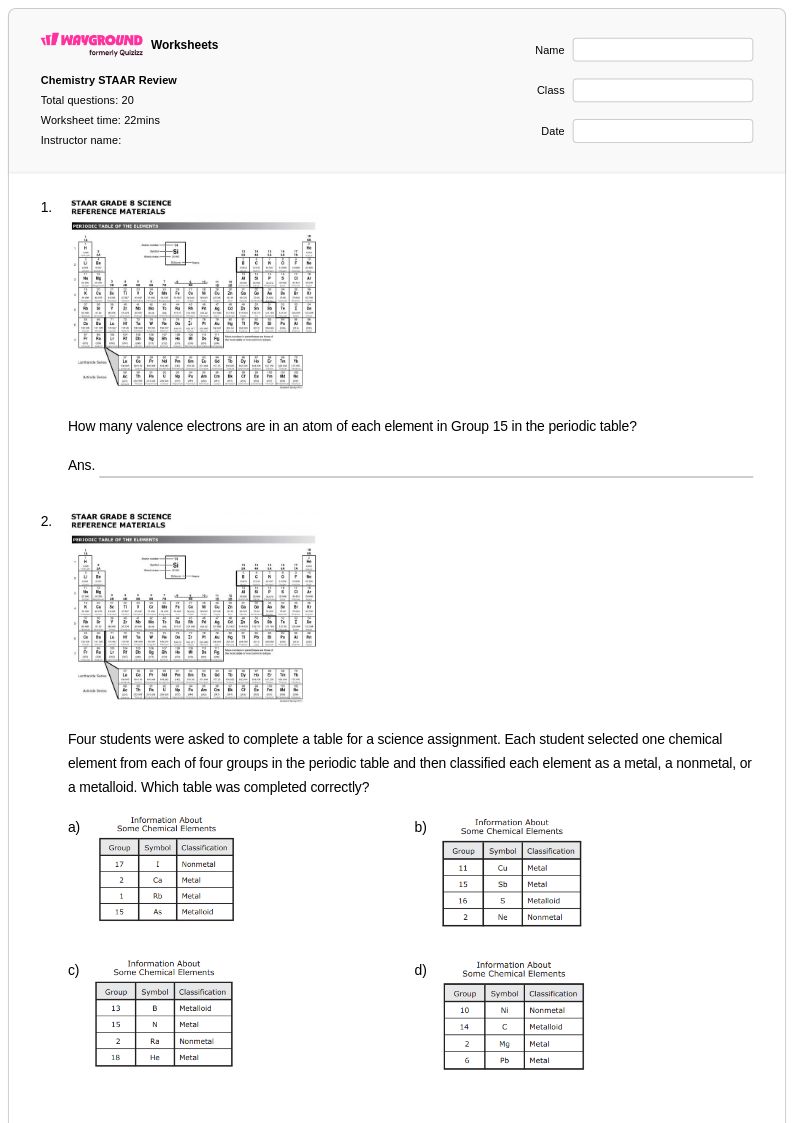
20 Q
7th

17 Q
7th
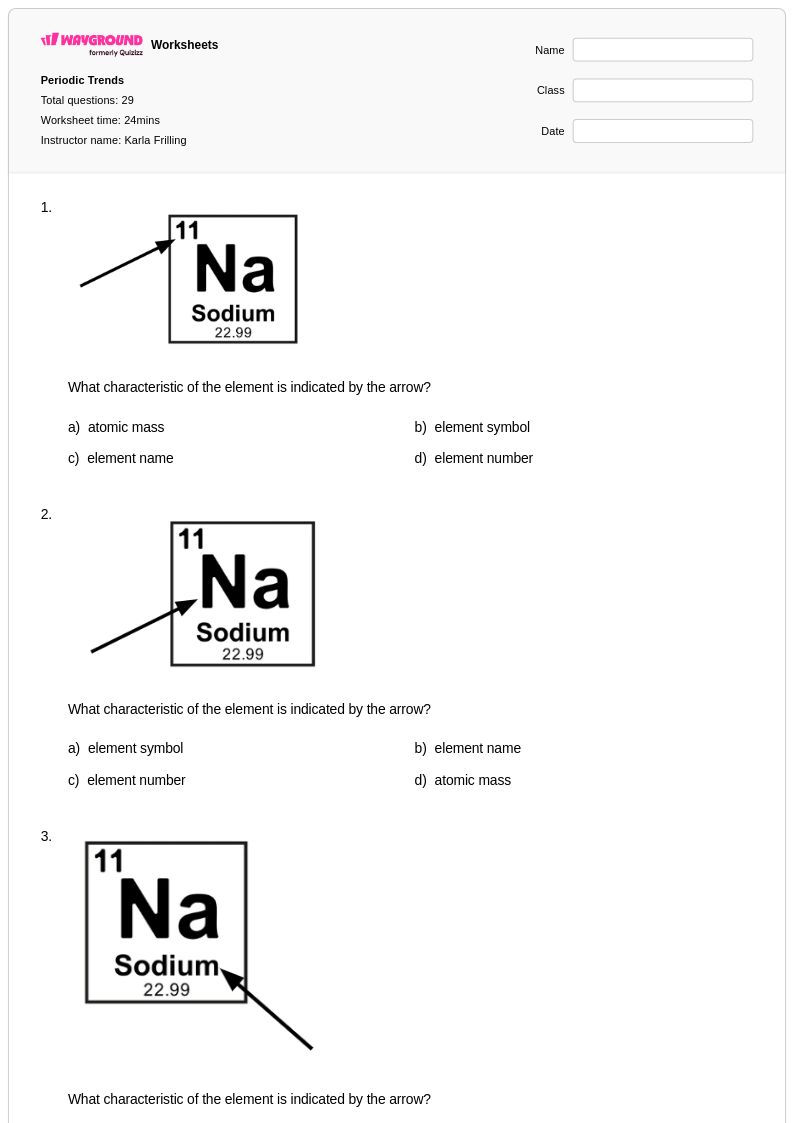
29 Q
7th

36 Q
7th
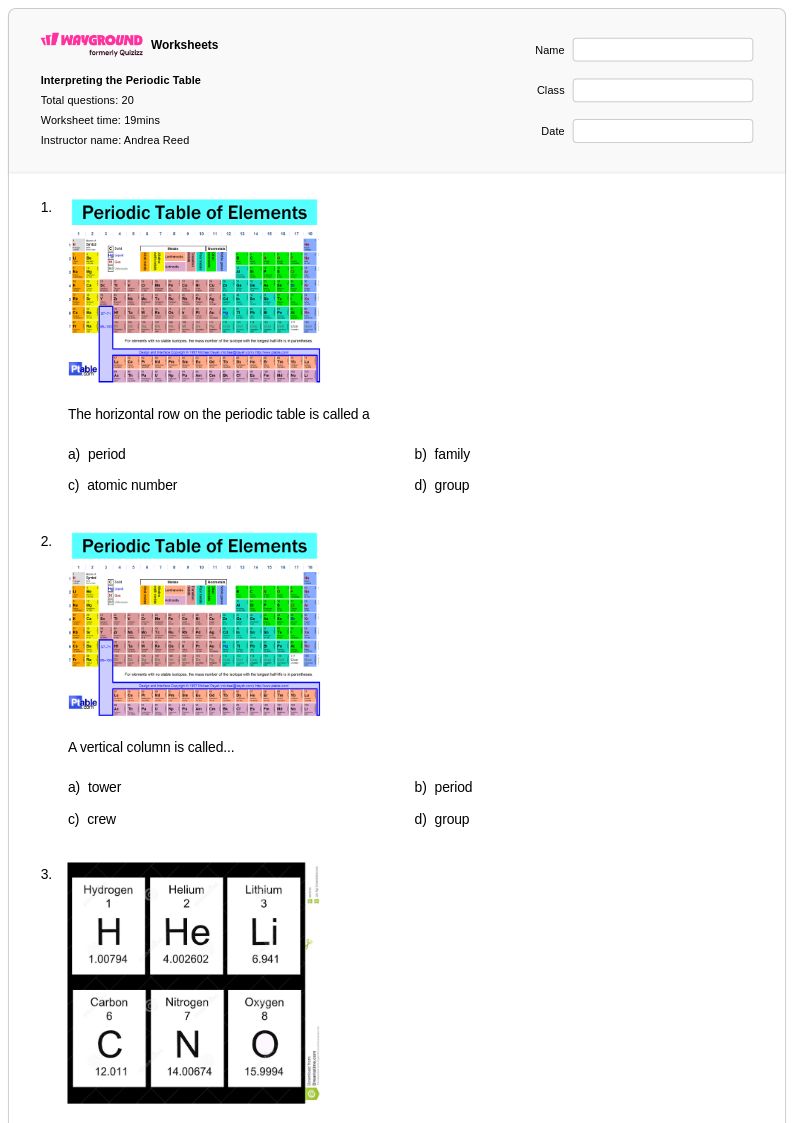
20 Q
6th - 8th

83 Q
7th

22 Q
6th - 8th
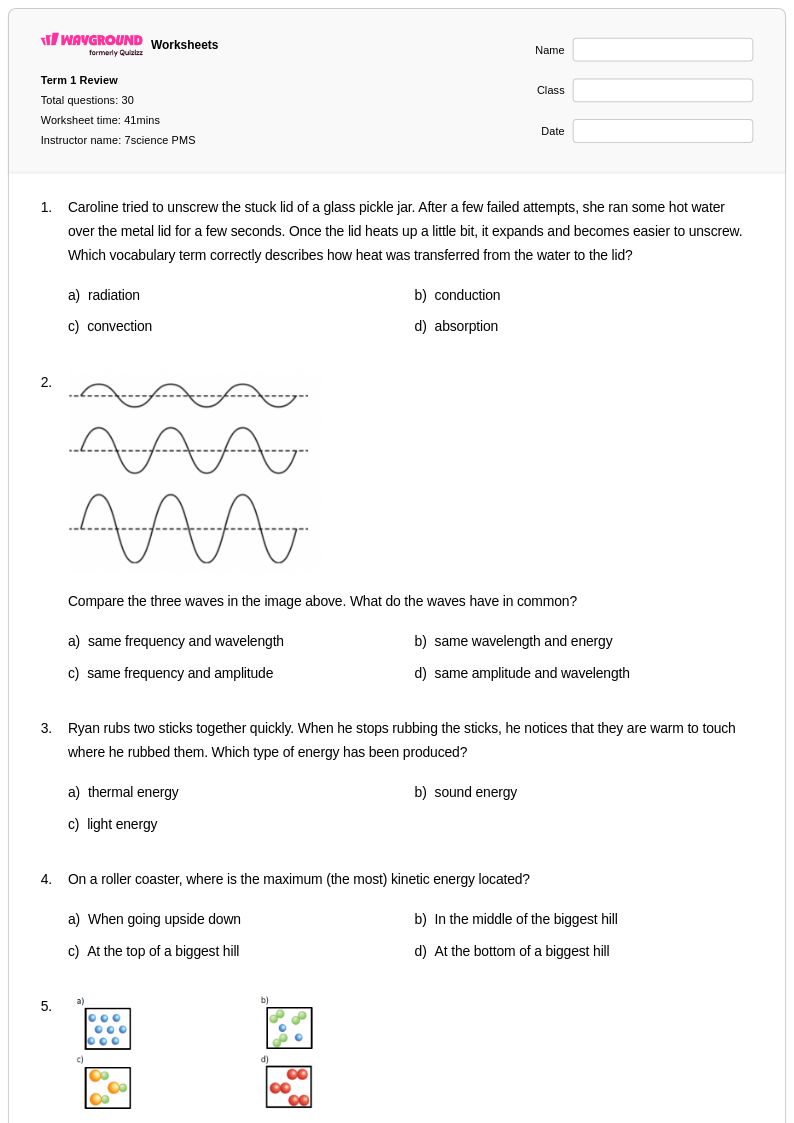
30 Q
7th
11 Q
6th - 8th

16 Q
6th - 8th
Explore Other Subject Worksheets for grade 7
Explore printable Valence Electrons worksheets for Grade 7
Valence electrons worksheets for Grade 7 students through Wayground (formerly Quizizz) provide comprehensive practice in understanding the outermost electrons that determine an element's chemical behavior and bonding patterns. These expertly designed resources strengthen essential chemistry skills including identifying valence electron configurations, predicting ionic charges, understanding periodic trends, and explaining how valence electrons influence chemical reactivity. Students work through carefully structured practice problems that build conceptual understanding from basic electron counting to more complex applications in chemical bonding. Each worksheet collection includes detailed answer keys to support independent learning and self-assessment, with free printable pdf options available to accommodate various classroom needs and homework assignments.
Wayground (formerly Quizizz) empowers teachers with millions of educator-created valence electron resources that streamline lesson planning and enhance student mastery of this fundamental chemistry concept. The platform's robust search and filtering capabilities allow instructors to quickly locate grade-appropriate materials aligned with science standards, while built-in differentiation tools enable customization for diverse learning needs and ability levels. Teachers can access these comprehensive worksheet collections in both printable and digital formats, including downloadable pdfs, making them ideal for in-class practice, homework assignments, remediation sessions, and enrichment activities. The extensive variety of problem types and difficulty levels supports targeted skill practice, helping educators address individual student needs while building a strong foundation in atomic structure and chemical bonding principles.
