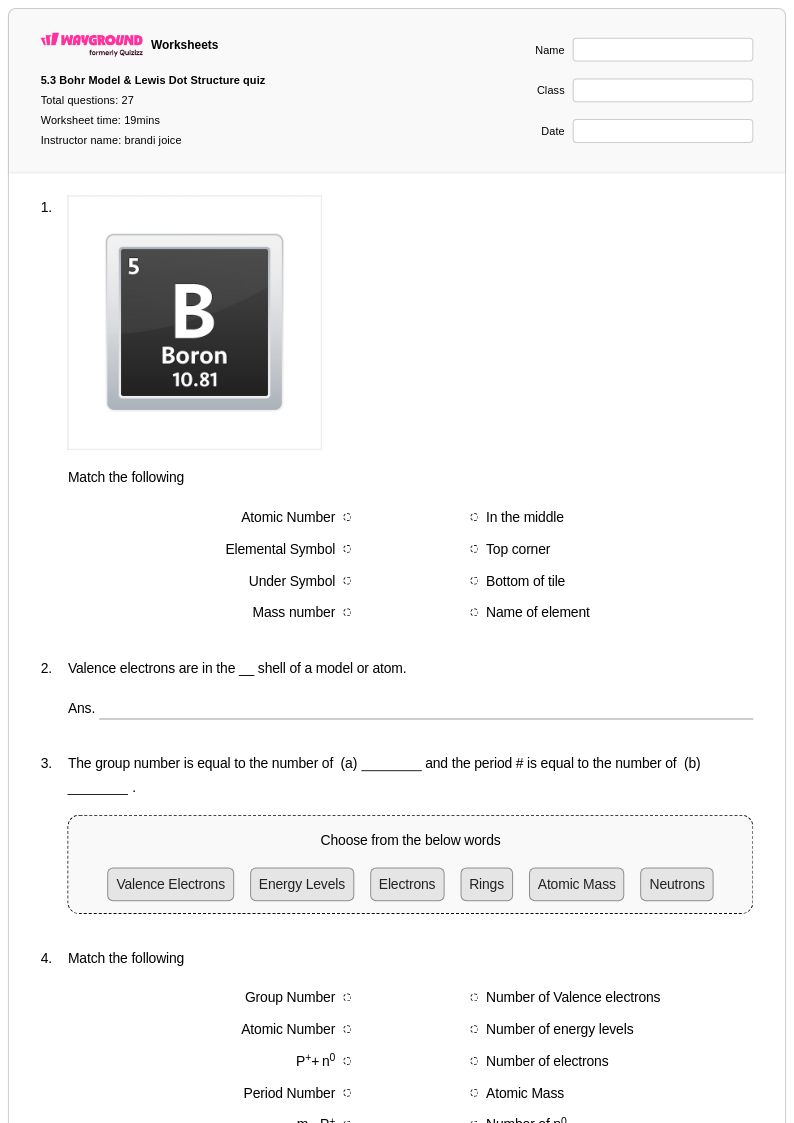
27Q
6th
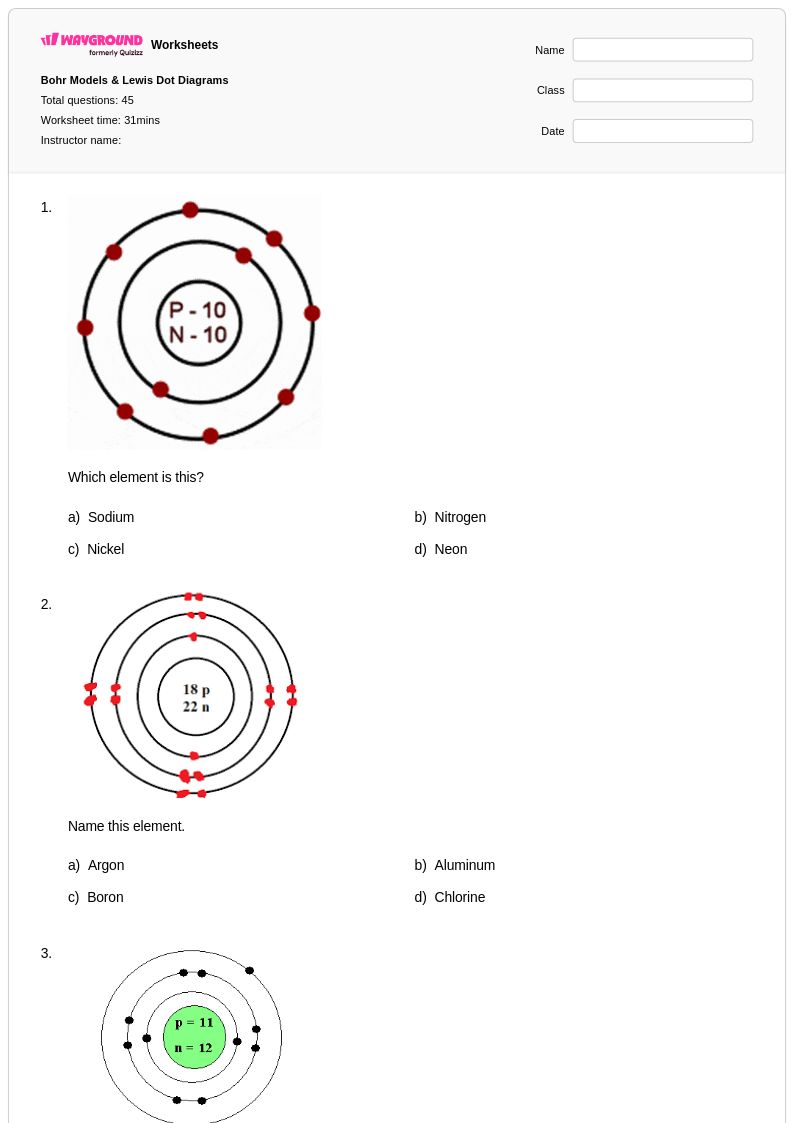
45Q
6th - 8th
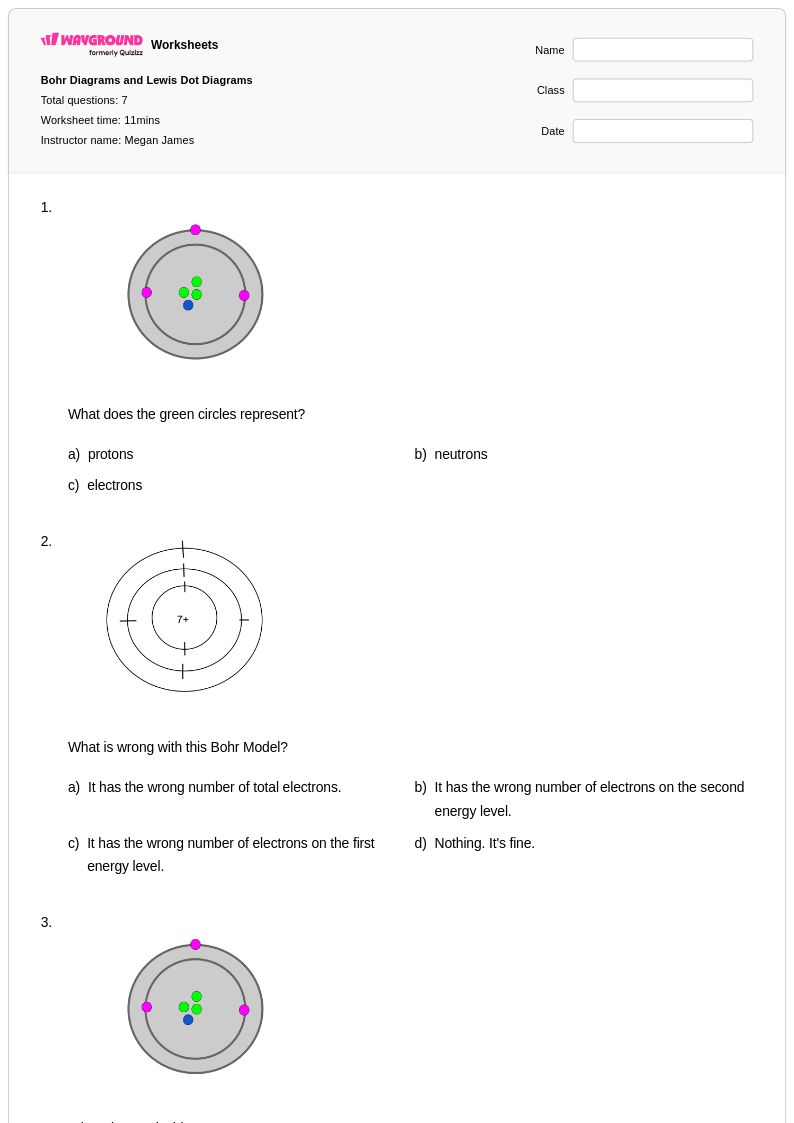
7Q
6th - 9th

36Q
6th - 8th
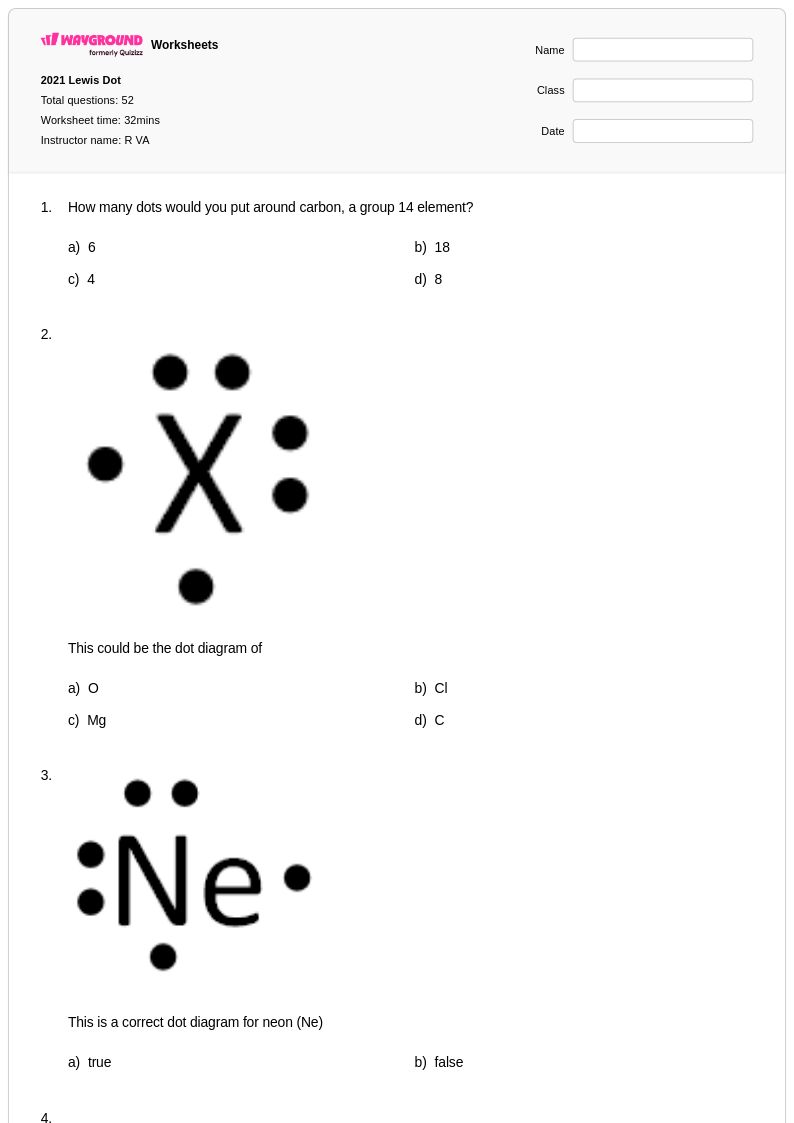
52Q
6th

25Q
6th - 8th
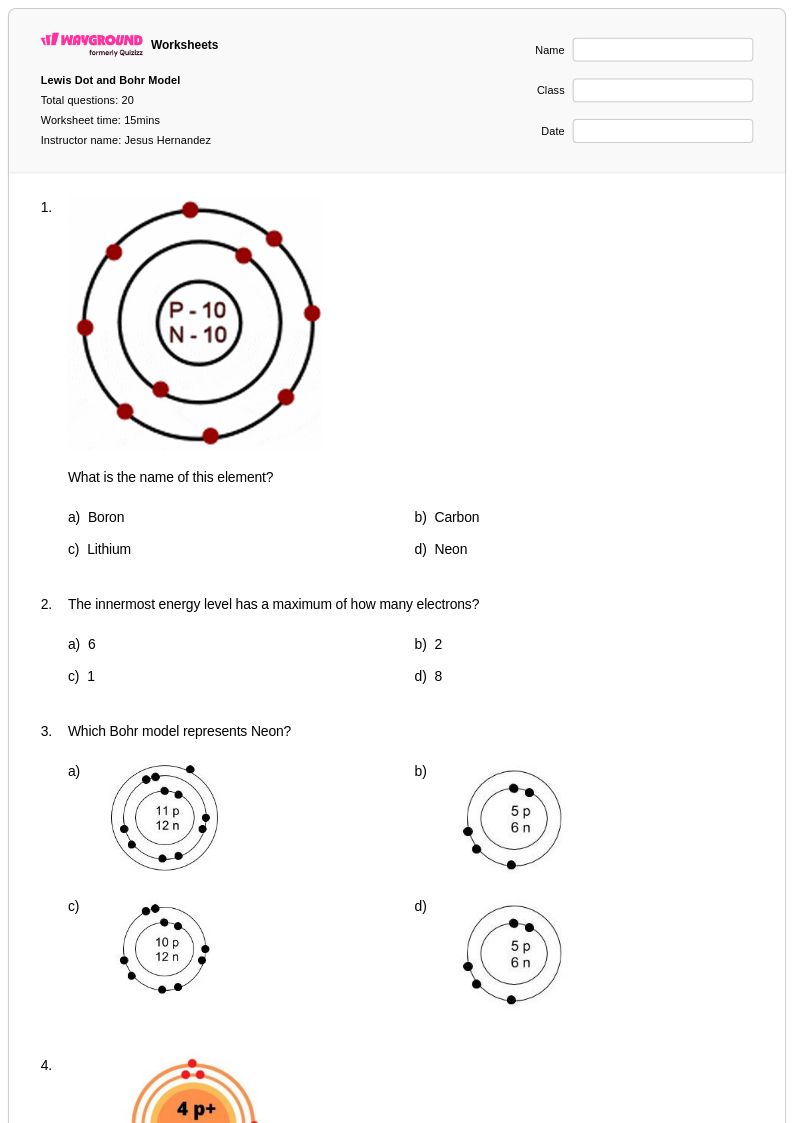
20Q
6th - 8th
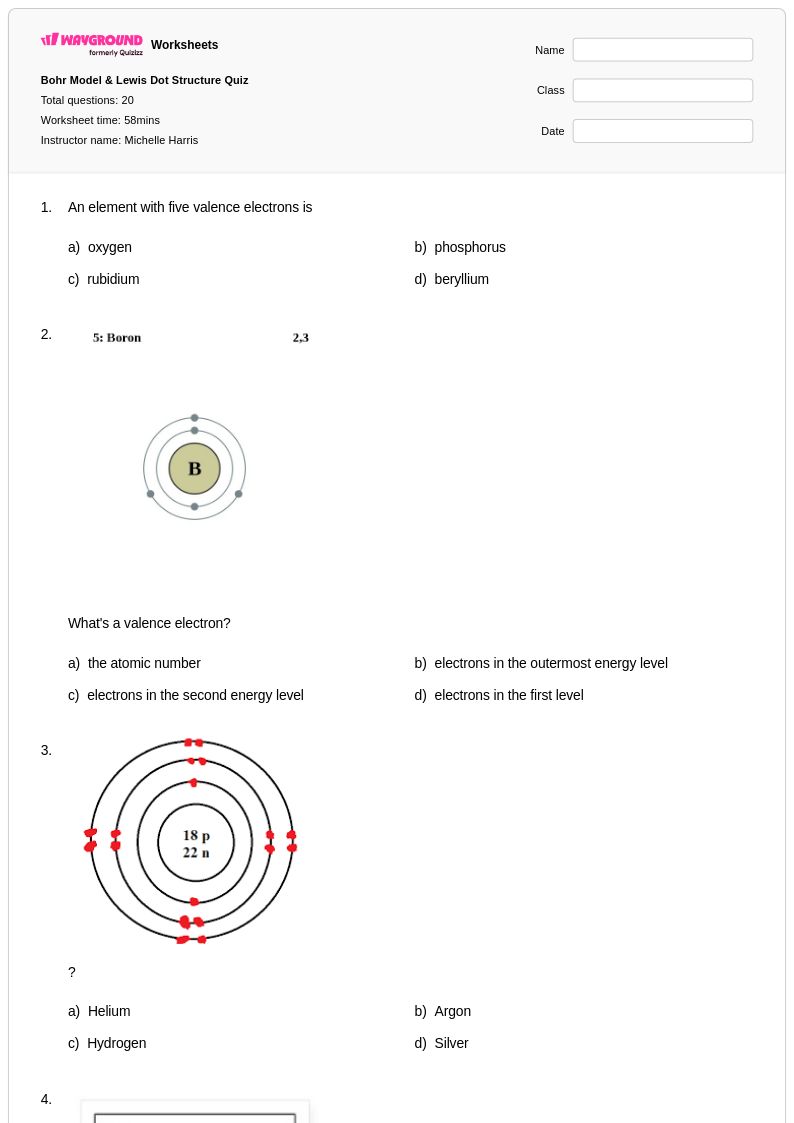
20Q
6th - 8th

60Q
6th - 8th
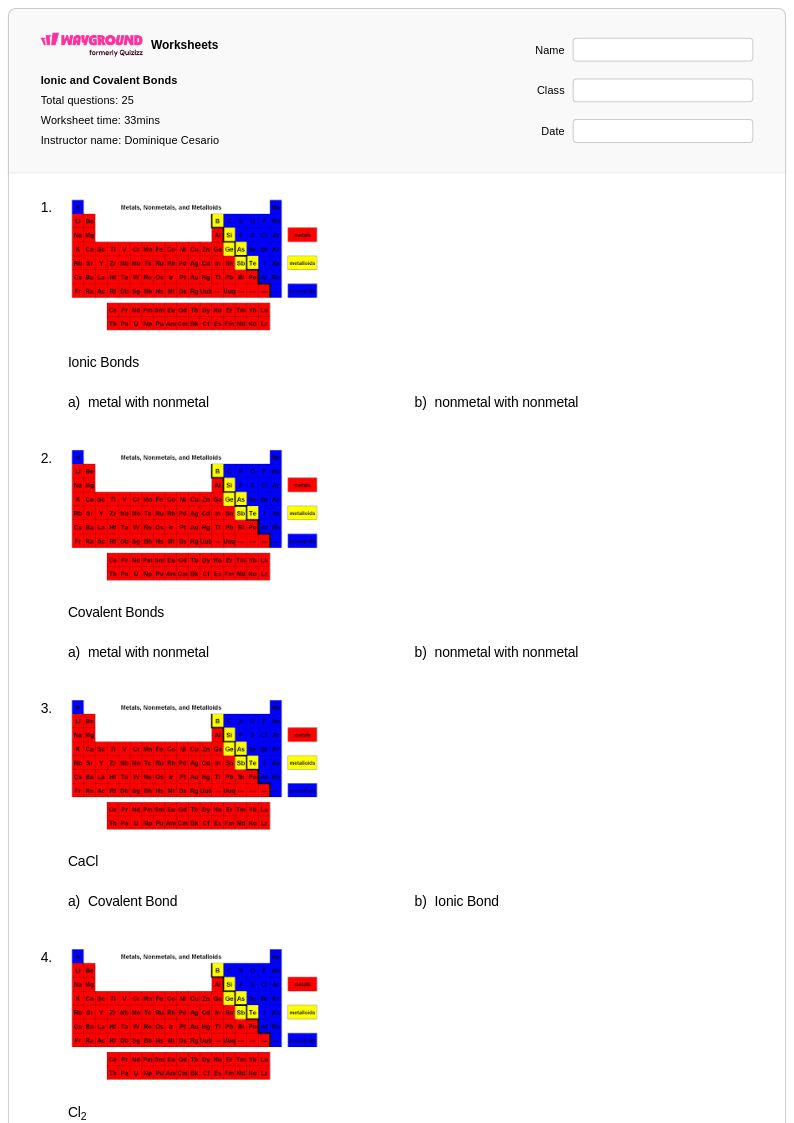
25Q
6th - 8th
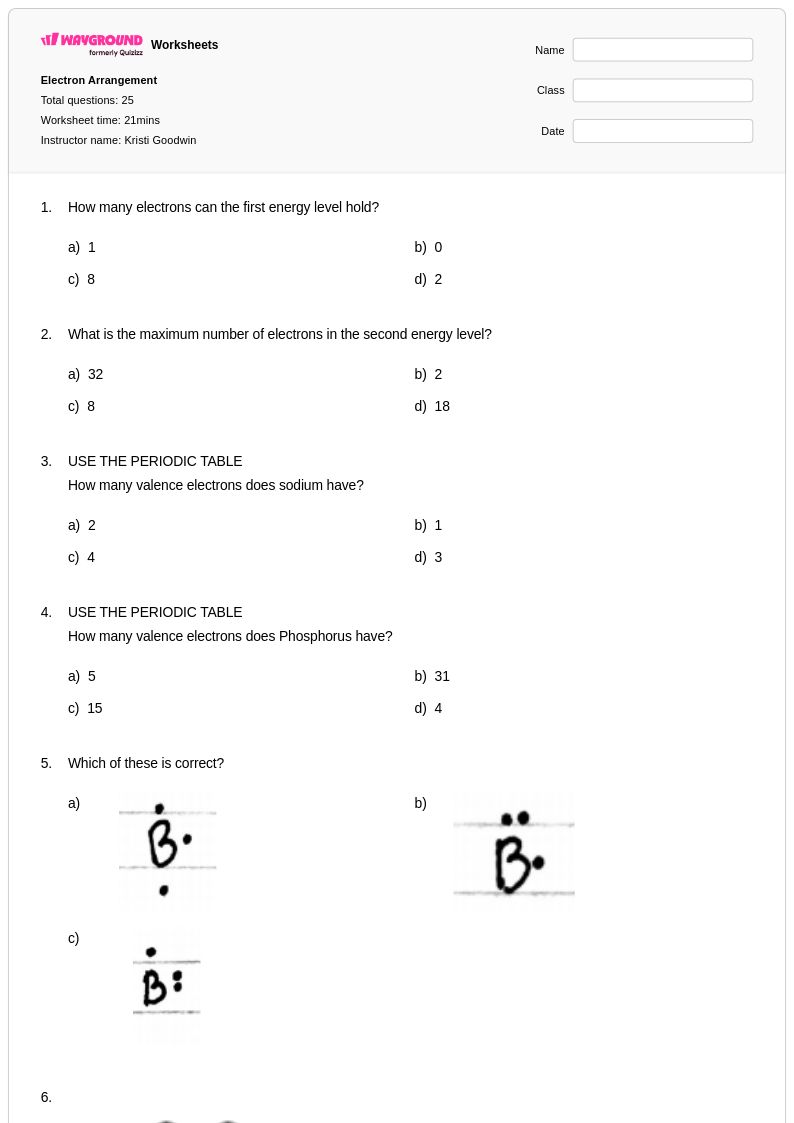
25Q
6th - 8th

30Q
6th - 8th
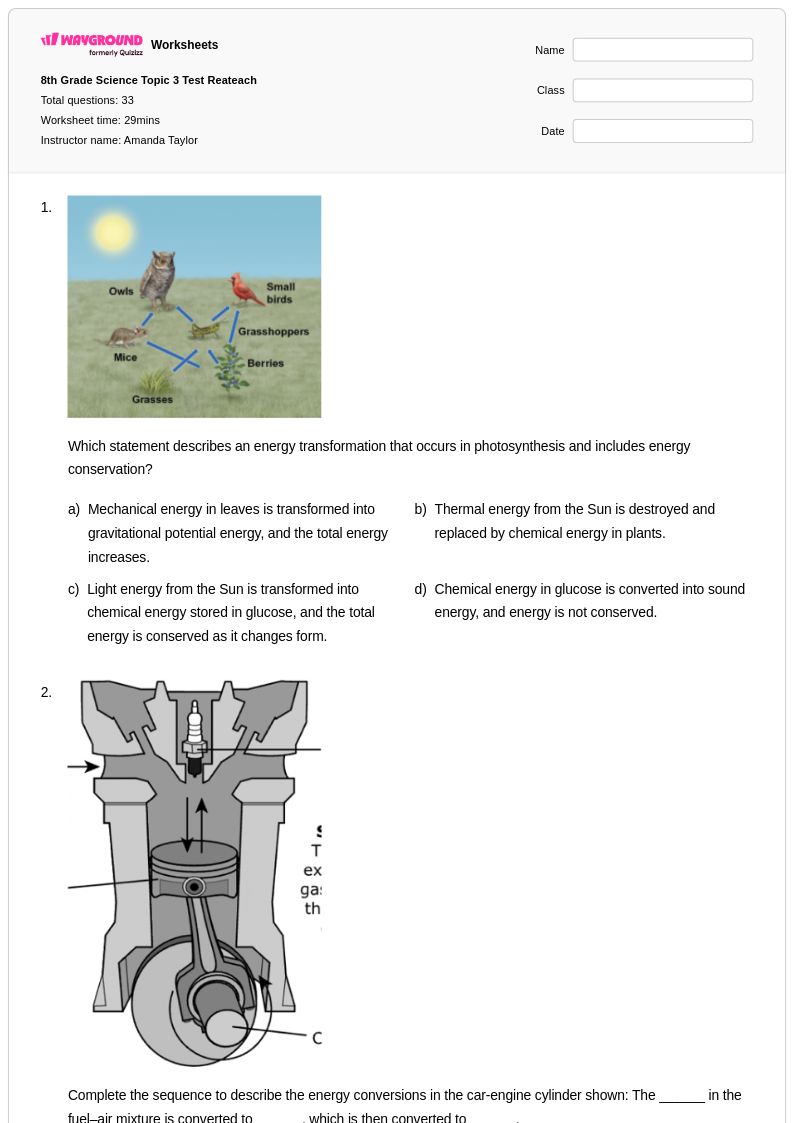
33Q
6th - 8th
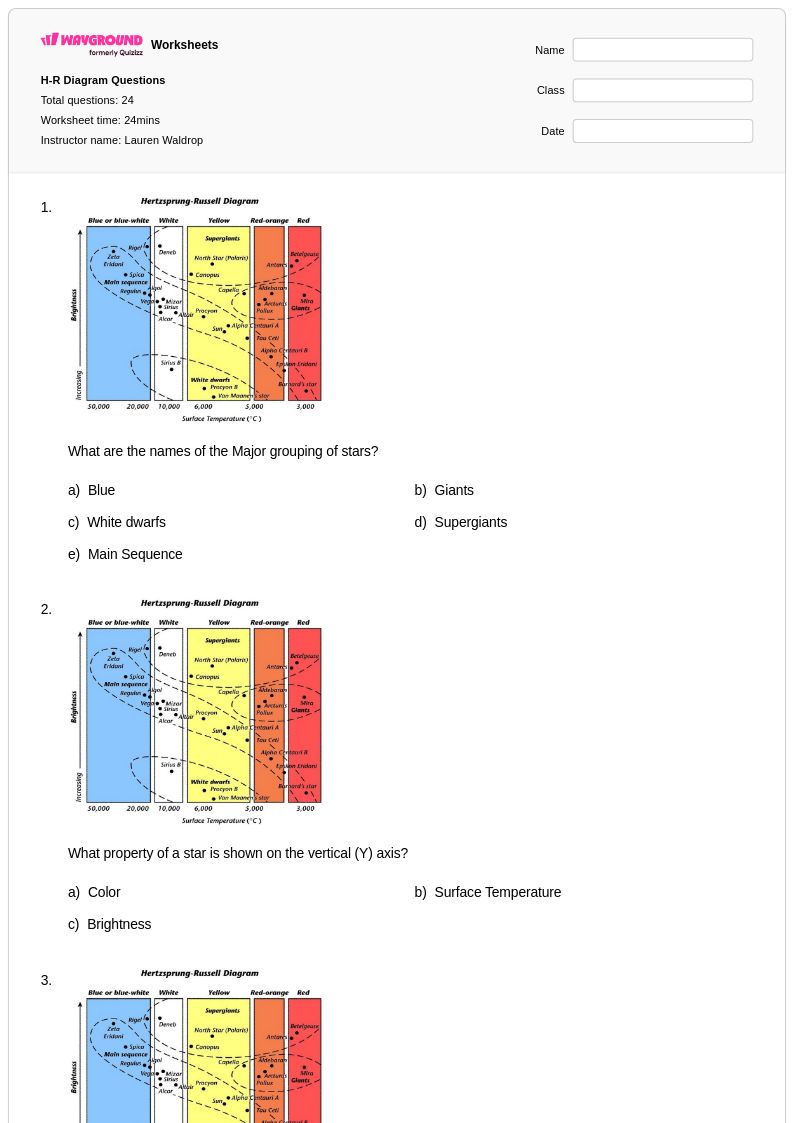
24Q
6th

15Q
6th

25Q
6th - 8th

17Q
6th - 8th
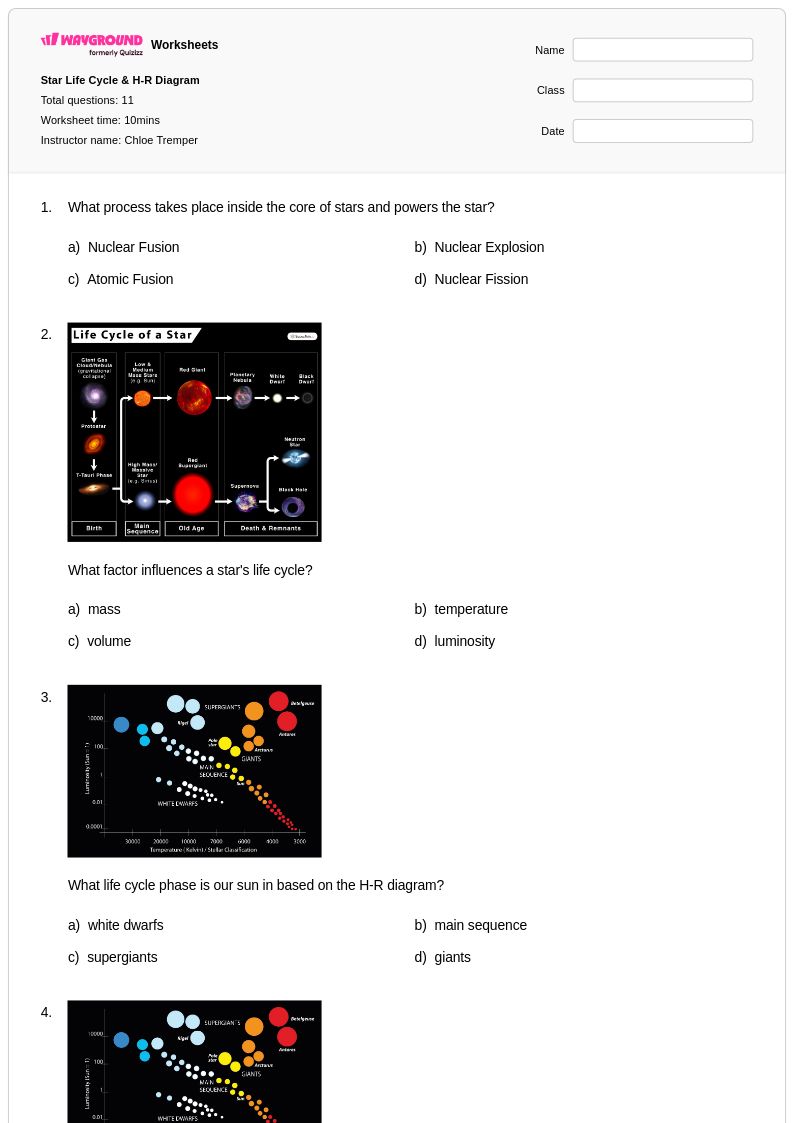
11Q
6th - 8th

75Q
6th - 8th

21Q
6th - 8th
16Q
6th - 8th
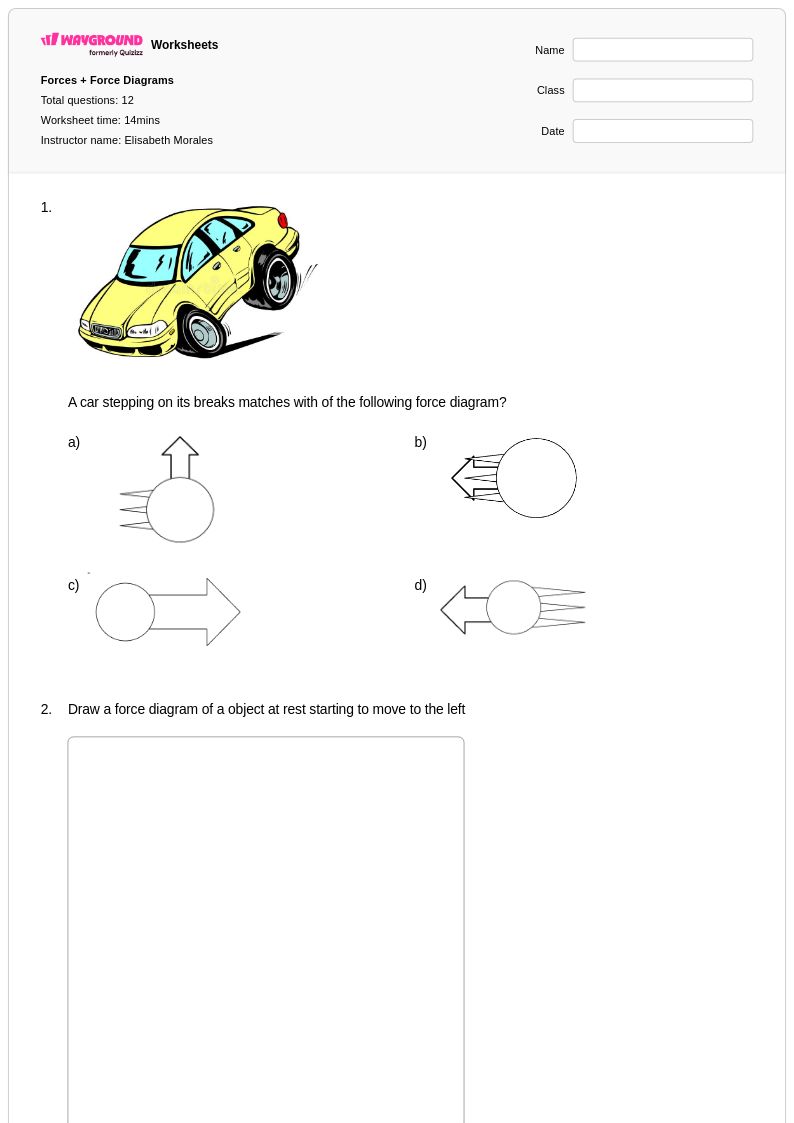
12Q
6th - 8th
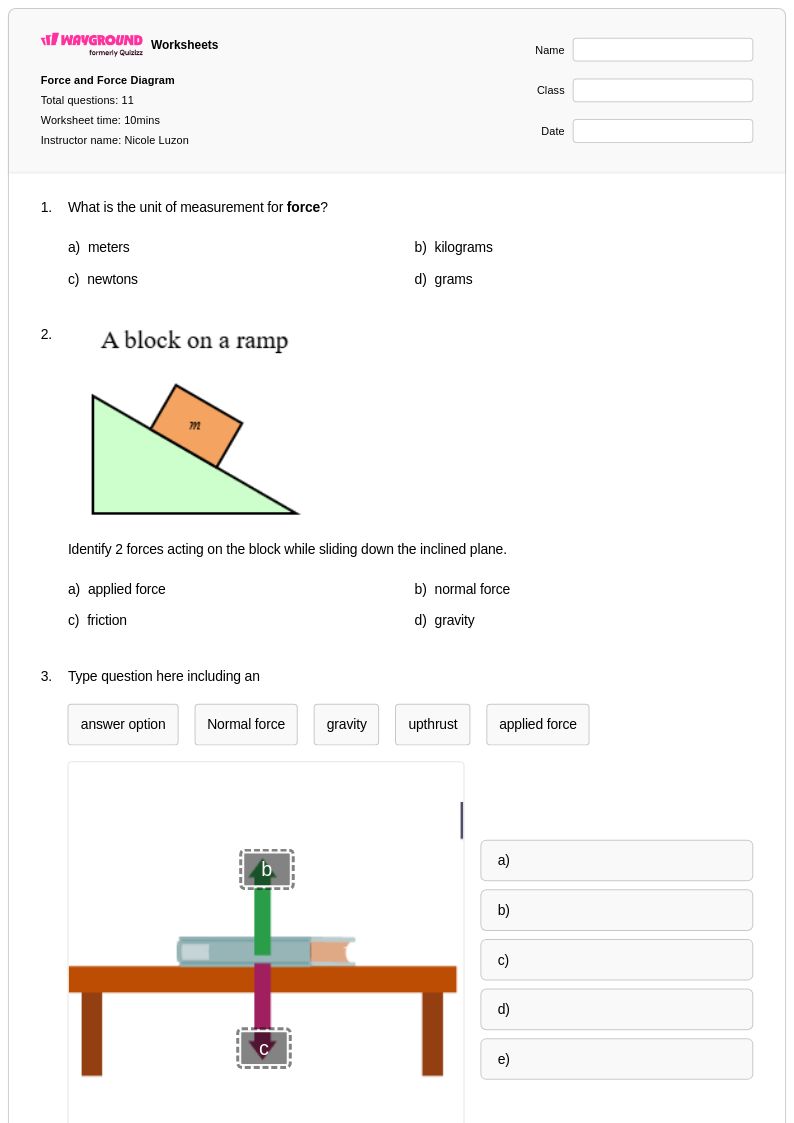
11Q
6th

15Q
6th
Explore outras planilhas de assuntos para year 6
Explore printable Lewis Dot Diagram worksheets for Year 6
Lewis dot diagrams provide Year 6 students with a foundational understanding of atomic structure and chemical bonding through visual representation of valence electrons. Wayground's comprehensive collection of Lewis dot diagram worksheets helps students master the essential skill of drawing electron configurations for atoms and simple molecules, building critical thinking abilities needed for advanced chemistry concepts. These carefully crafted practice problems guide students through step-by-step processes for determining valence electrons, placing dots around element symbols, and understanding how atoms achieve stability. The free printable worksheets include detailed answer keys that support independent learning and self-assessment, while pdf formats ensure consistent formatting across different devices and printing options.
Wayground (formerly Quizizz) empowers educators with millions of teacher-created Lewis dot diagram resources specifically designed for Year 6 science instruction. The platform's advanced search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific learning standards and differentiate instruction based on individual student needs. These customizable digital and printable materials support diverse classroom applications, from initial concept introduction and guided practice to remediation for struggling learners and enrichment activities for advanced students. Teachers can seamlessly modify existing worksheets or combine multiple resources to create comprehensive lesson plans that address varying skill levels, ensuring every student develops confidence in drawing Lewis structures and understanding electron behavior in atoms and molecules.
