12 Q
6th - 8th
10 Q
6th

60 Q
6th - 8th
16 Q
6th - 8th
25 Q
6th - 8th

37 Q
6th - 8th
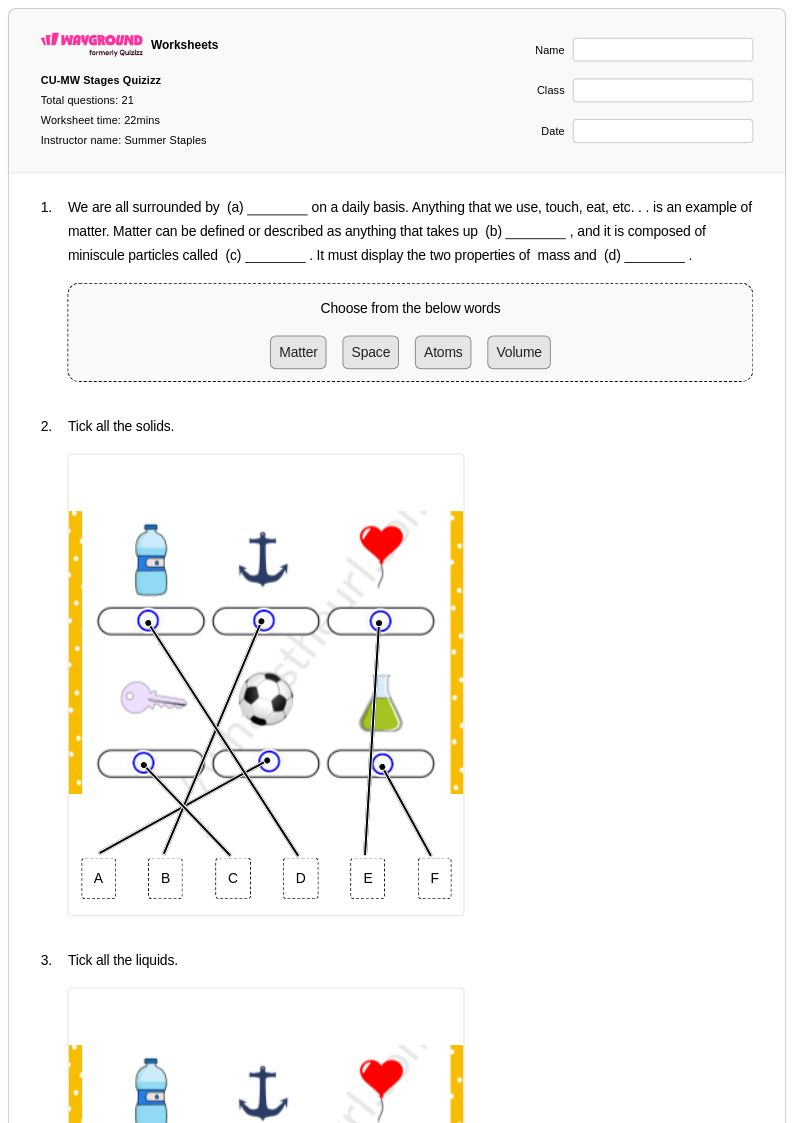
21 Q
6th - 8th
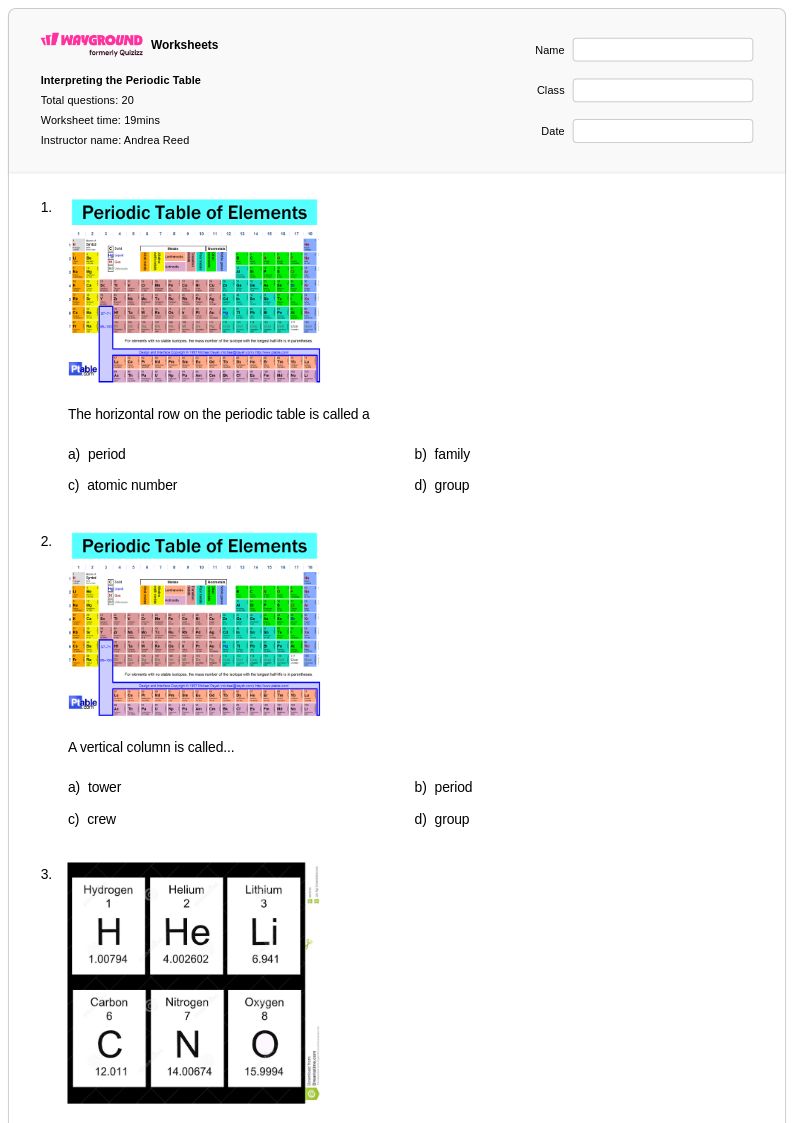
20 Q
6th - 8th

95 Q
6th - 8th

69 Q
6th - 10th

22 Q
6th - 8th
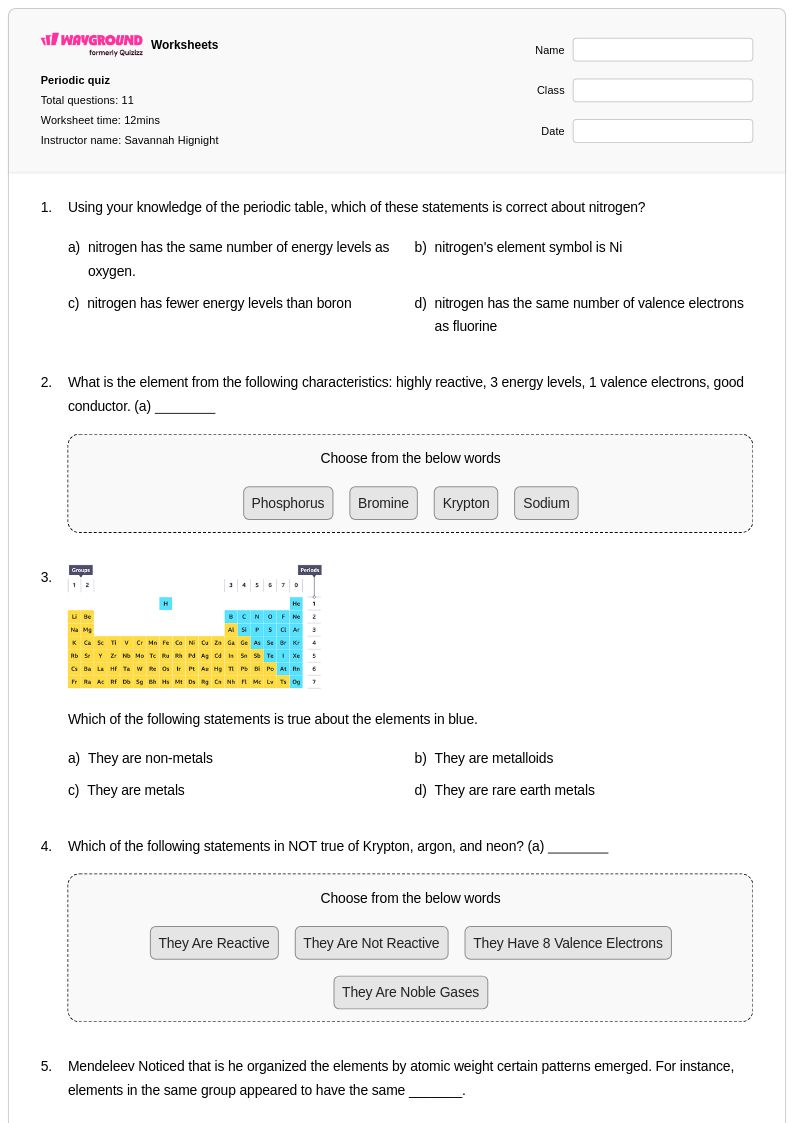
11 Q
6th - 8th

16 Q
6th - 8th
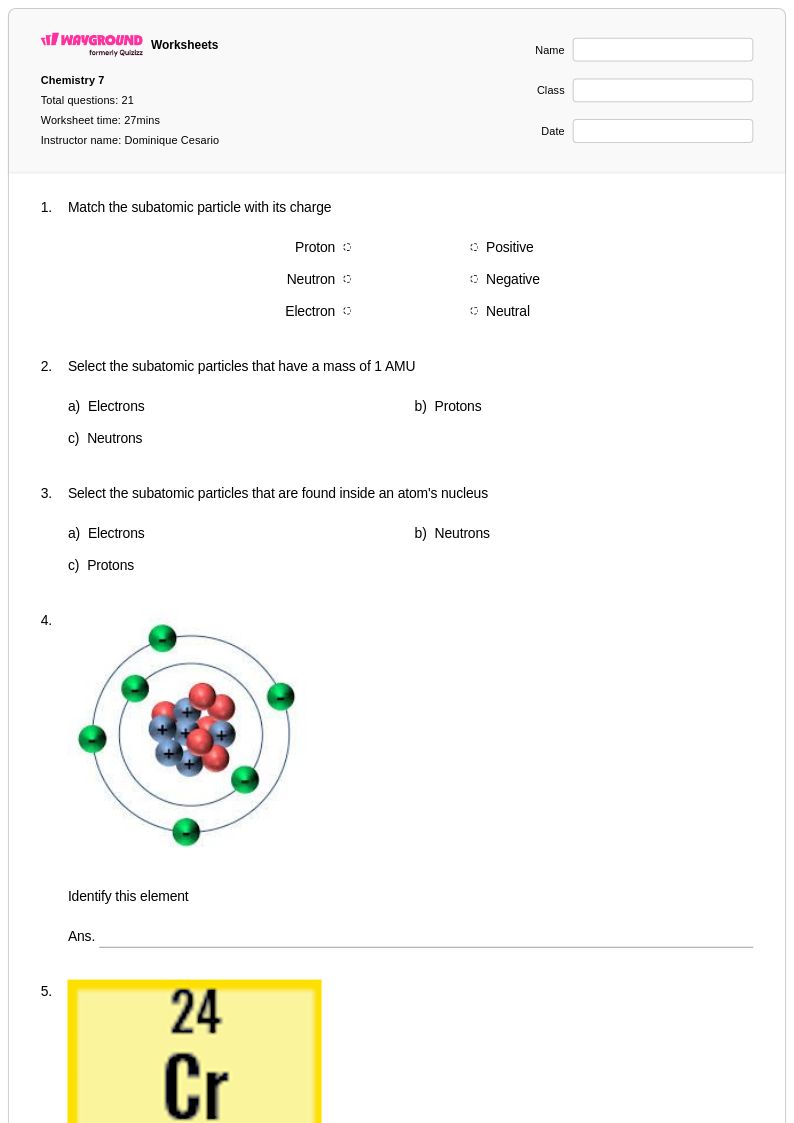
21 Q
6th - 8th
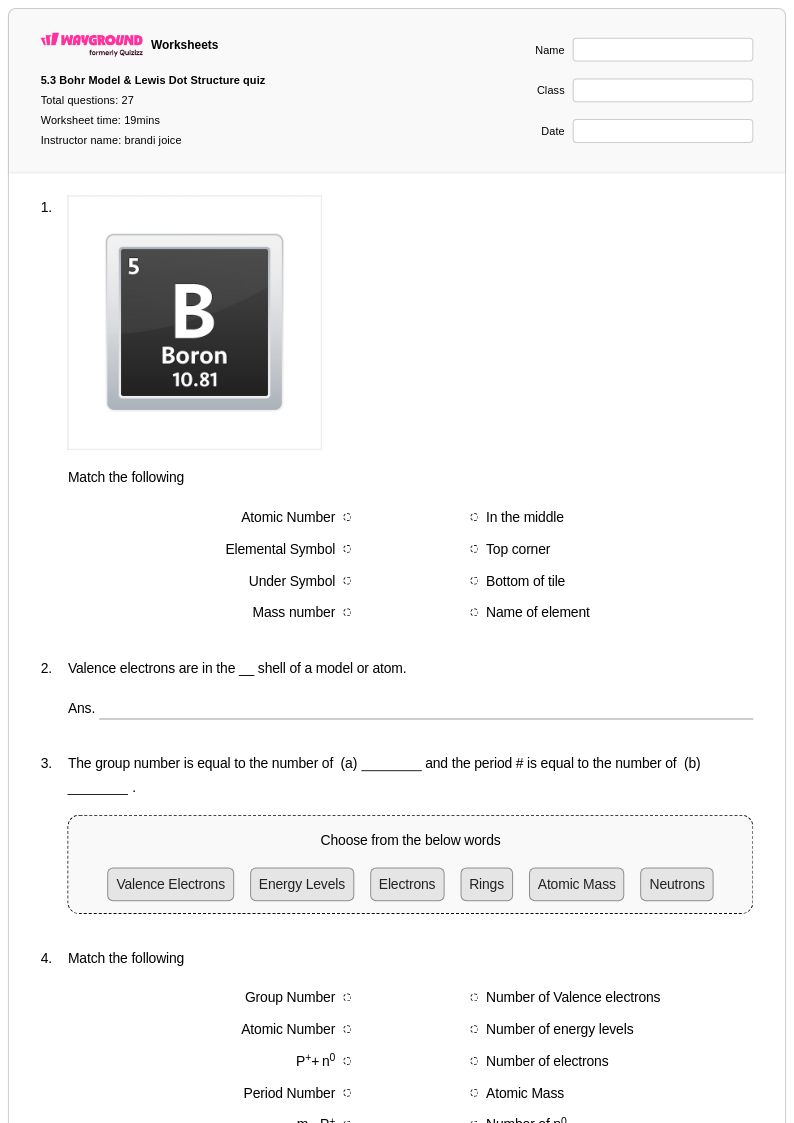
27 Q
6th

21 Q
6th - 8th
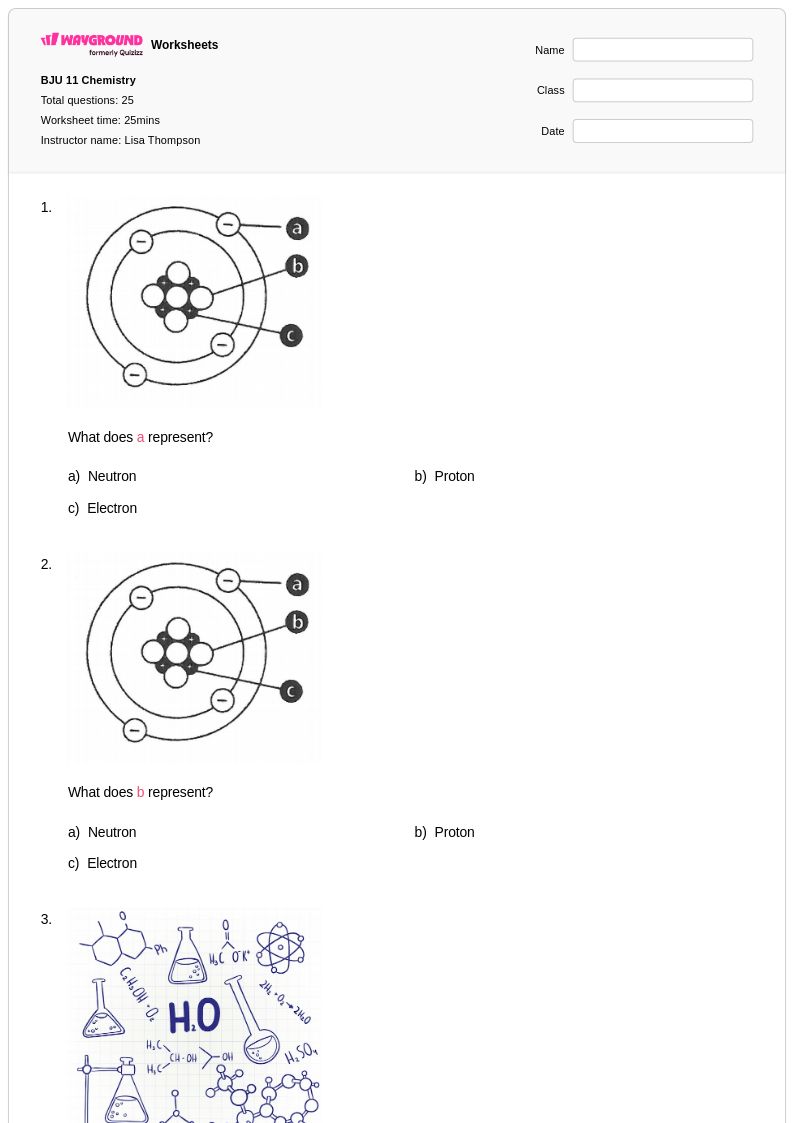
25 Q
6th - Uni

16 Q
6th

27 Q
6th - 8th

10 Q
6th - 9th

15 Q
6th - 8th
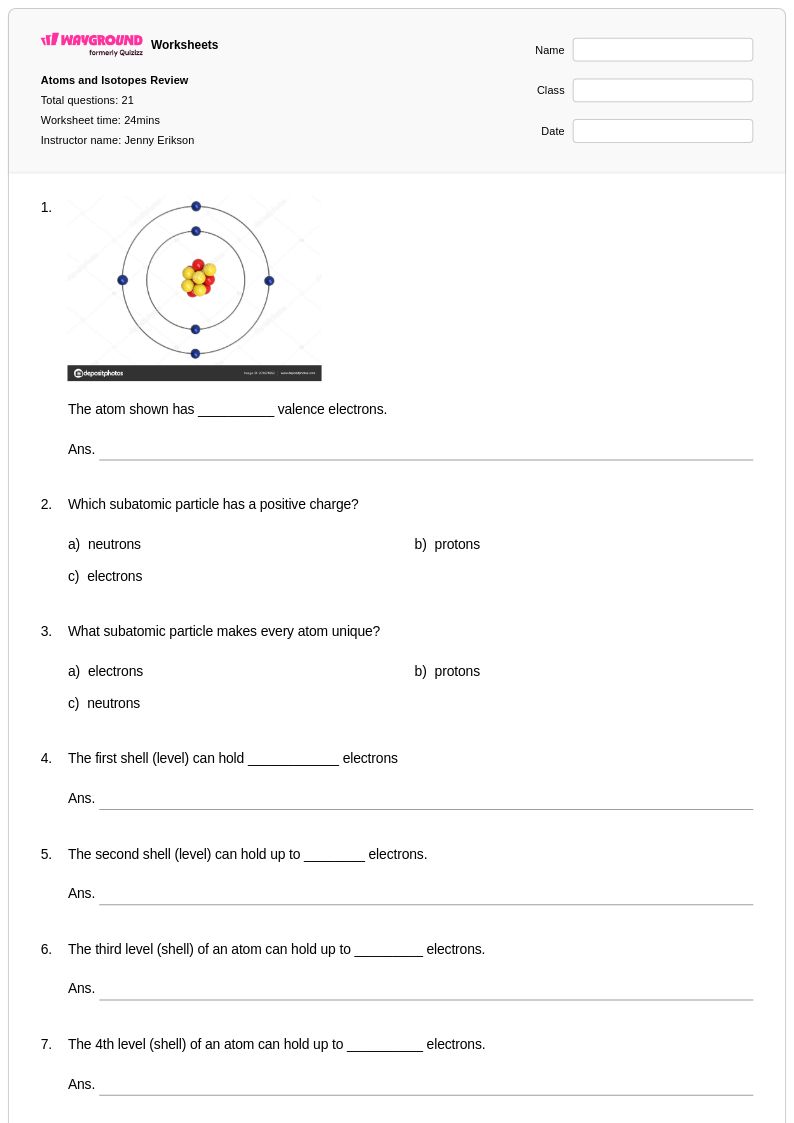
21 Q
6th - 8th
18 Q
6th
Explore Other Subject Worksheets for year 6
Explore printable Valence Electrons worksheets for Year 6
Valence electrons worksheets for Year 6 chemistry provide students with essential practice in understanding how atoms form bonds and interact with other elements. These comprehensive worksheet collections available through Wayground (formerly Quizizz) focus on helping sixth-grade students master the concept of valence electrons - the outermost electrons that determine an atom's chemical behavior. Students work through carefully designed practice problems that teach them to identify valence electrons using the periodic table, understand electron dot diagrams, and predict how atoms will bond based on their electron configurations. Each worksheet includes detailed answer keys and explanations, making these free printable resources invaluable for both classroom instruction and independent study, while pdf formats ensure easy distribution and consistent formatting across different devices and printing systems.
Wayground (formerly Quizizz) empowers teachers with millions of educator-created valence electron worksheets and chemistry resources that streamline lesson planning and provide targeted skill practice for Year 6 students. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets that align with specific curriculum standards and match their students' varying ability levels, while built-in differentiation tools enable customization of difficulty and content focus. Teachers can access these resources in both printable pdf format for traditional paper-and-pencil work and digital formats for interactive learning experiences, making it simple to incorporate valence electron practice into remediation sessions, enrichment activities, or regular classroom instruction. The extensive collection ensures that educators have access to diverse problem types and teaching approaches, supporting comprehensive understanding of this fundamental chemistry concept that serves as a building block for more advanced topics in chemical bonding and molecular structure.
