
30Q
11th

20Q
9th - 12th

43Q
10th - Uni
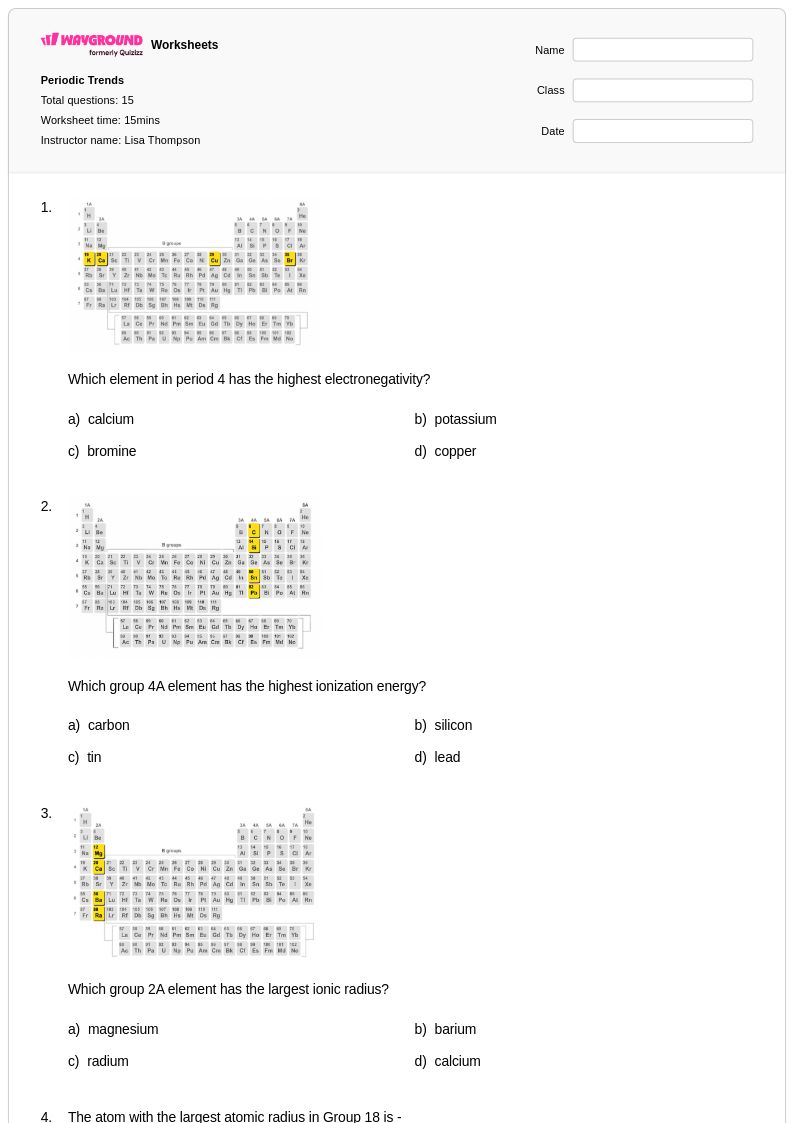
15Q
10th - Uni
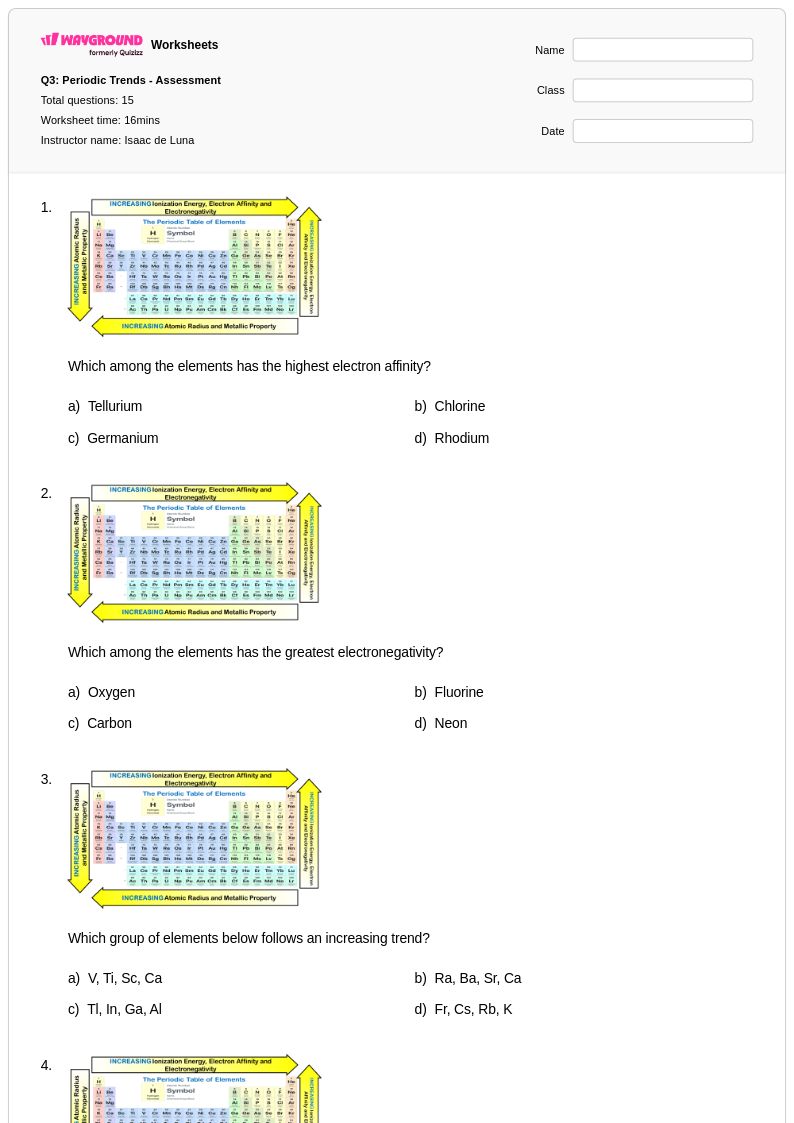
15Q
8th
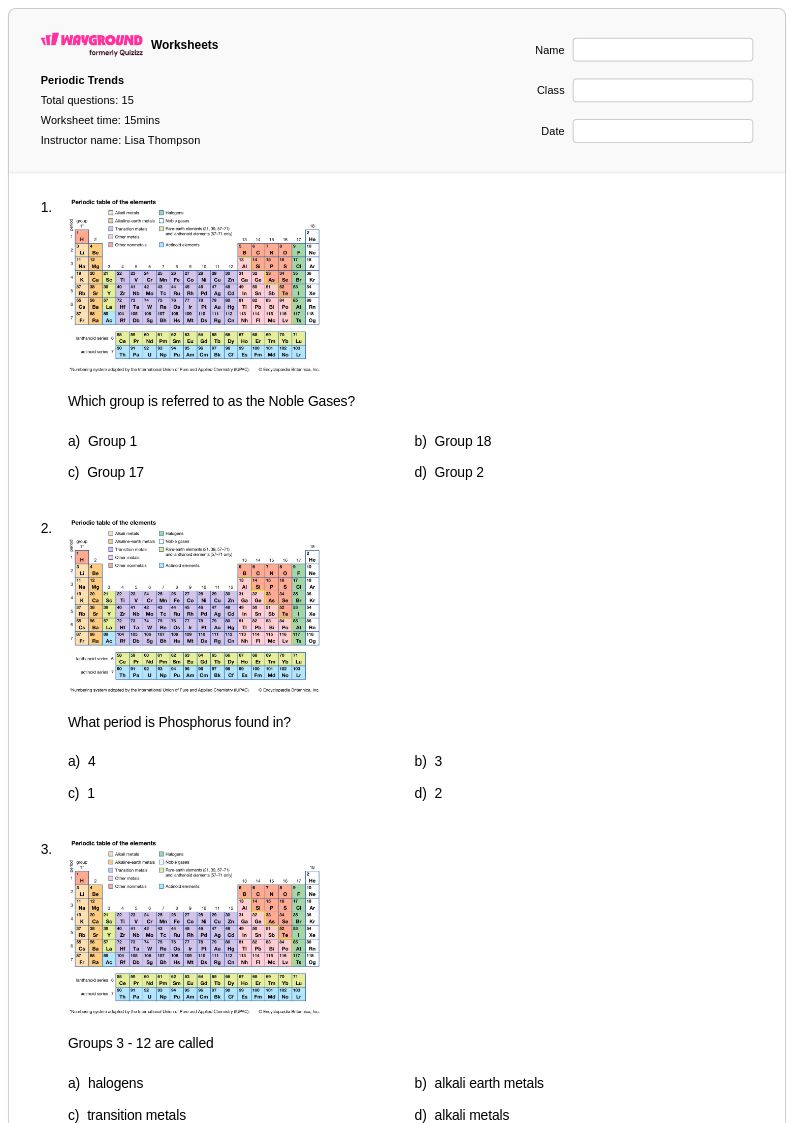
15Q
10th - Uni
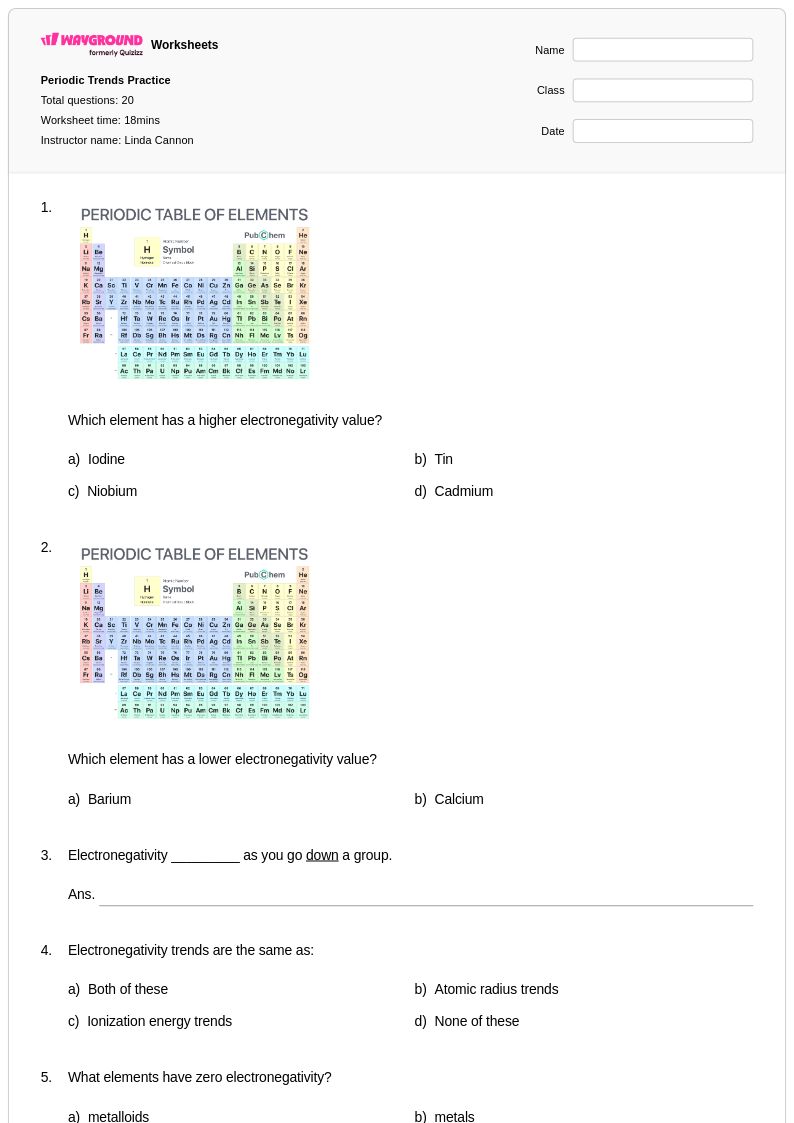
20Q
10th - 12th

14Q
10th

25Q
9th - 12th

21Q
4th - Uni

42Q
10th

56Q
11th
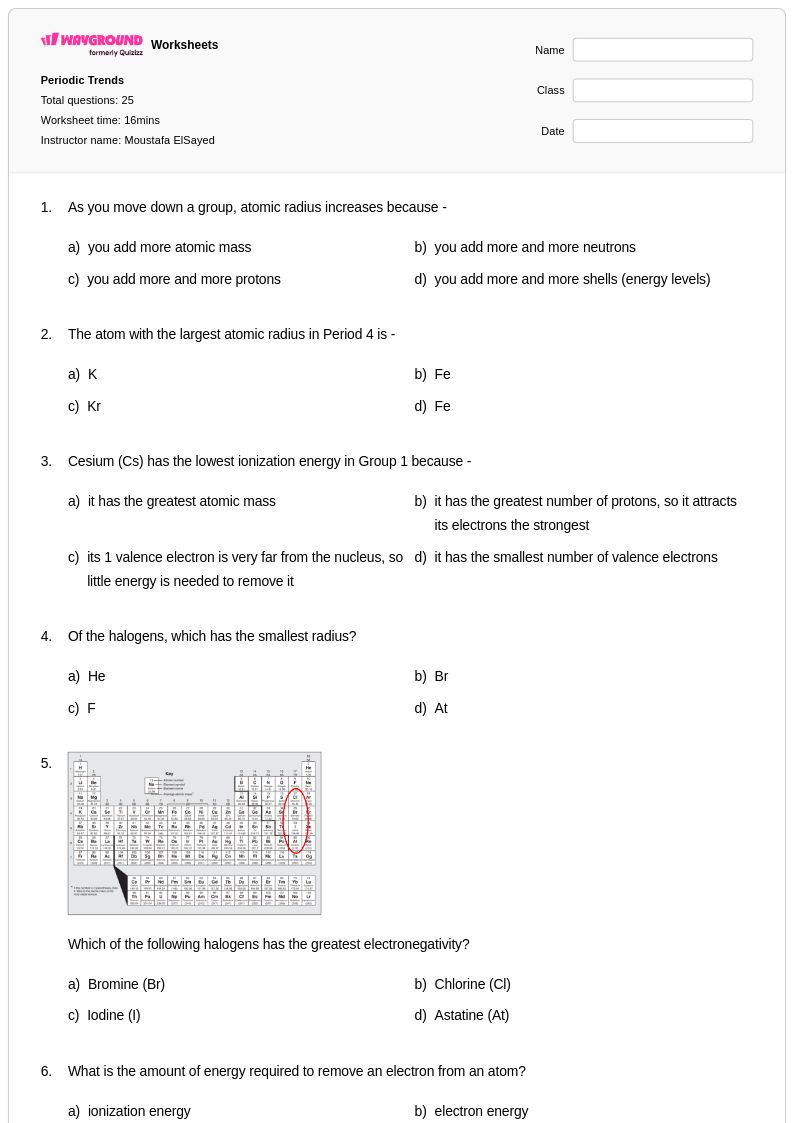
25Q
9th - 12th

15Q
10th

27Q
10th

16Q
12th
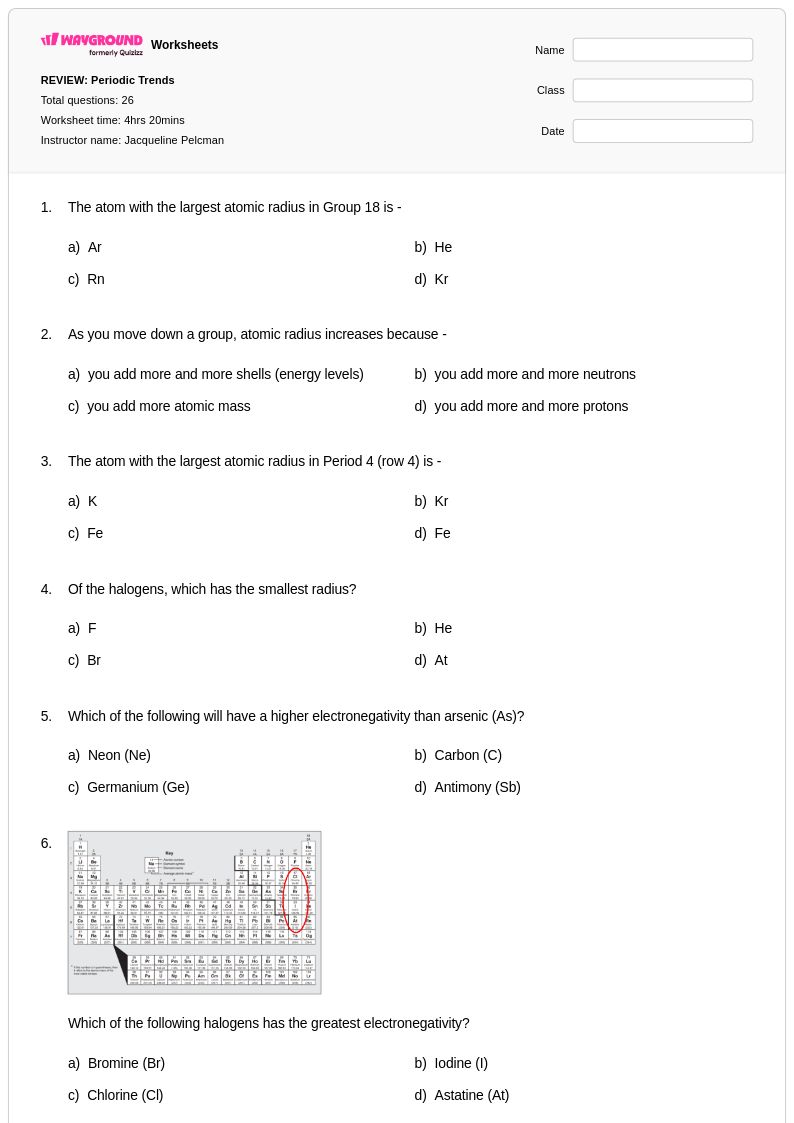
26Q
10th
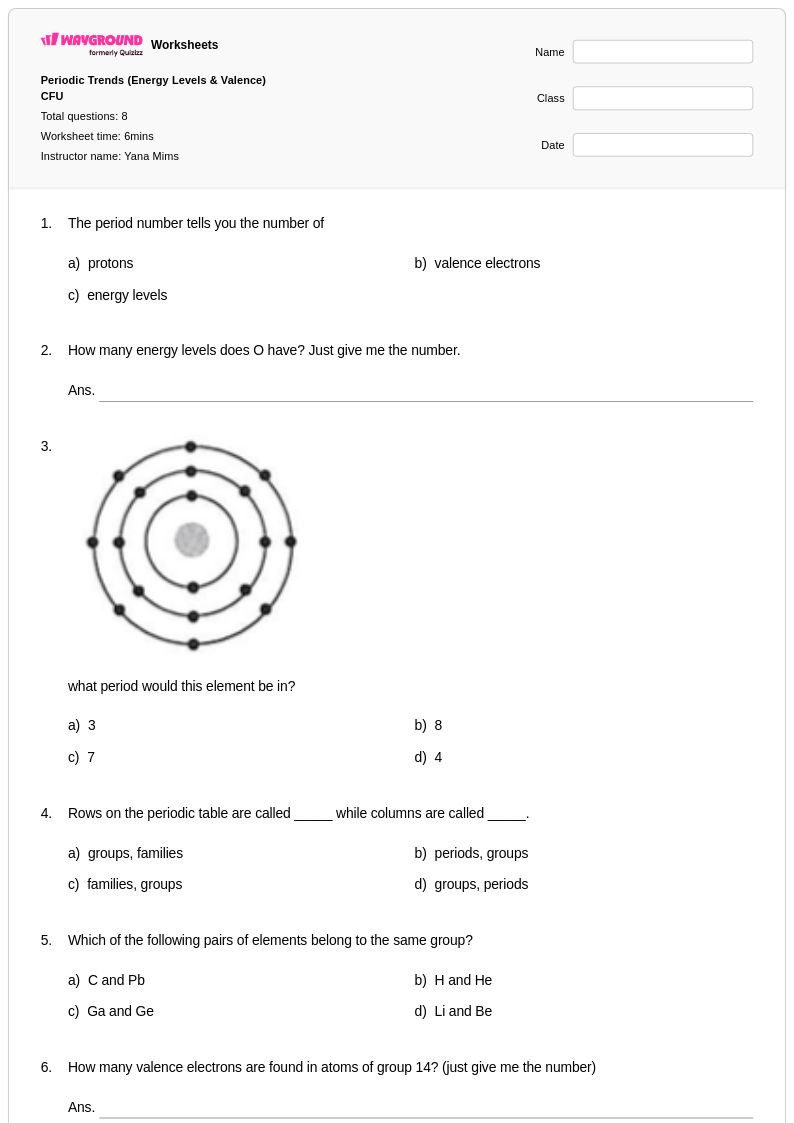
8Q
10th - 12th

15Q
12th

15Q
6th - 10th
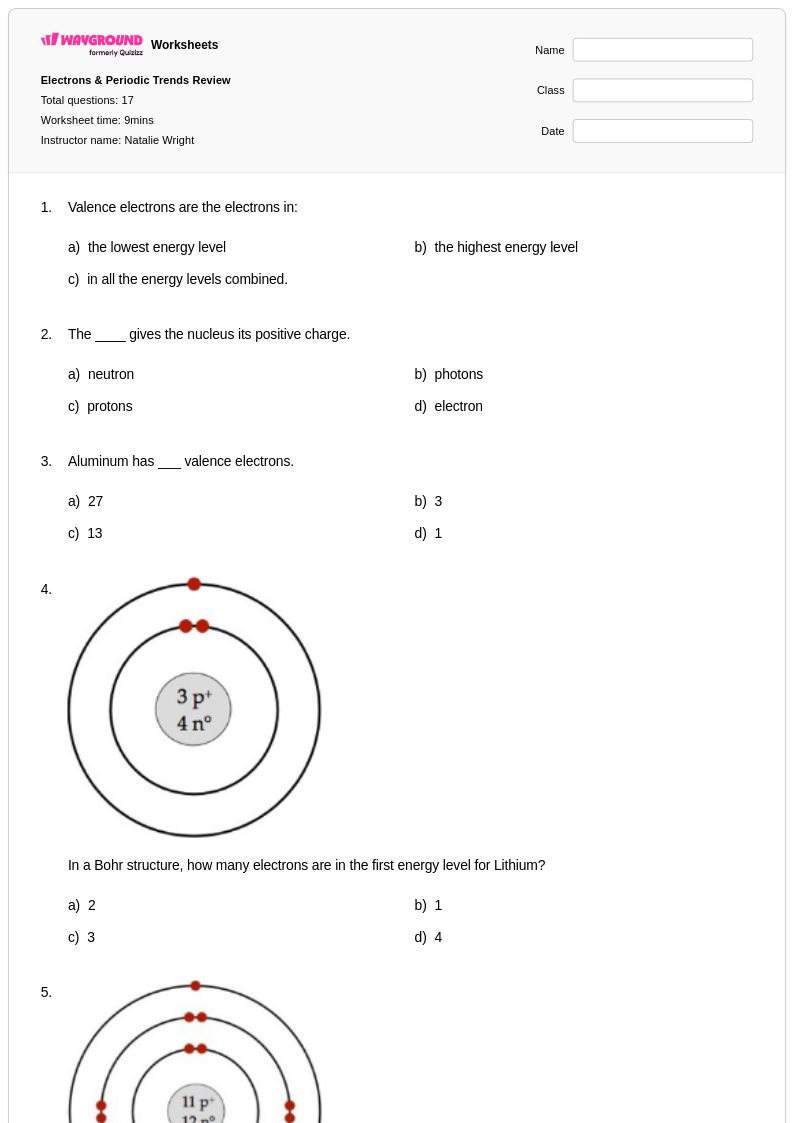
17Q
9th

25Q
10th - Uni

23Q
10th

20Q
10th
Explorar hojas de trabajo por materias
Explore printable Periodic Trends worksheets
Periodic trends worksheets available through Wayground (formerly Quizizz) provide comprehensive practice materials that help students master the predictable patterns found across the periodic table of elements. These chemistry resources focus on developing critical analytical skills as students explore how atomic radius, ionization energy, electronegativity, and metallic character change systematically across periods and down groups. The worksheets feature carefully structured practice problems that guide learners through interpreting periodic trends using electron configuration principles and effective nuclear charge concepts. Each printable resource includes detailed answer keys that support independent study and self-assessment, while the free pdf format ensures easy classroom distribution and homework assignments that reinforce fundamental chemistry concepts.
Wayground (formerly Quizizz) empowers chemistry educators with an extensive collection of millions of teacher-created periodic trends resources that streamline lesson planning and differentiated instruction. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets that align with specific chemistry standards and match their students' varying skill levels. These customizable materials support both remediation for struggling learners and enrichment opportunities for advanced students, with flexible formatting options that accommodate both digital classroom environments and traditional printable assignments. The comprehensive worksheet collections enable teachers to provide targeted skill practice that builds conceptual understanding of periodic law applications, electron shielding effects, and the relationship between atomic structure and chemical properties across all elements in the periodic table.
