
18 Q
9th - 12th
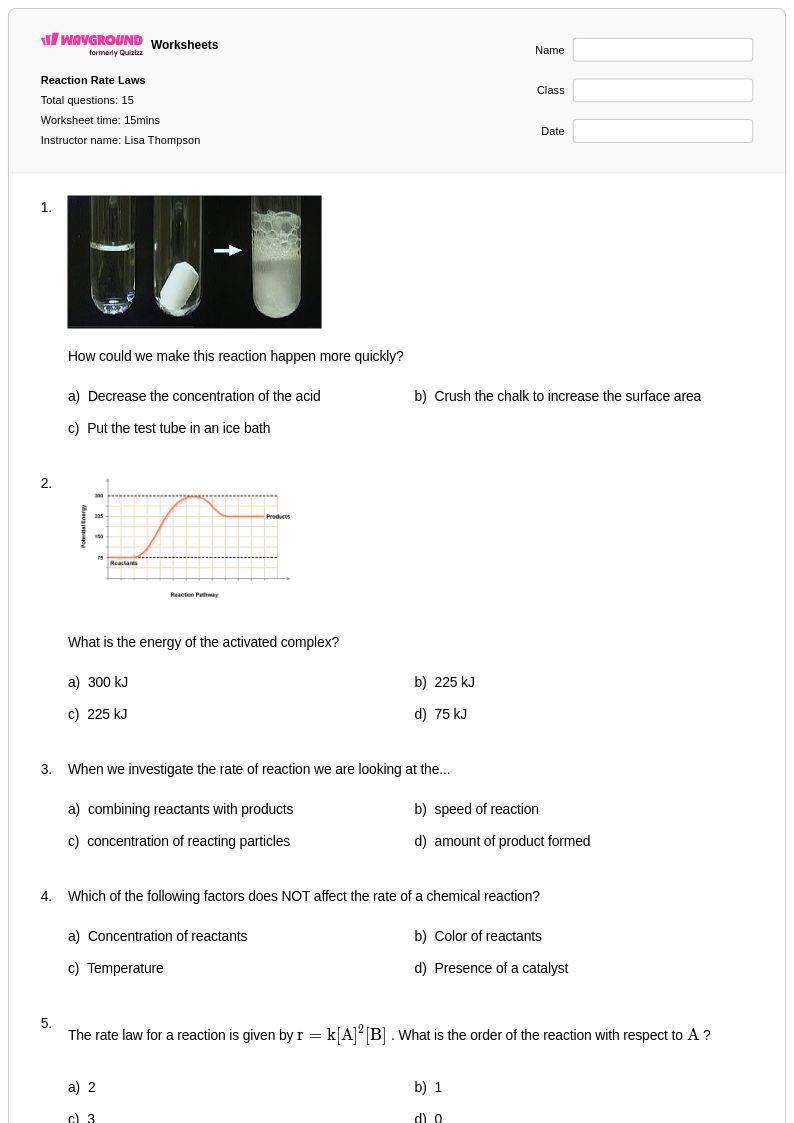
15 Q
8th - Uni
25 Q
8th - Uni

15 Q
8th - Uni

13 Q
7th - 9th
14 Q
9th

19 Q
9th - 11th

15 Q
8th - Uni

8 Q
7th - 10th
12 Q
7th - Uni
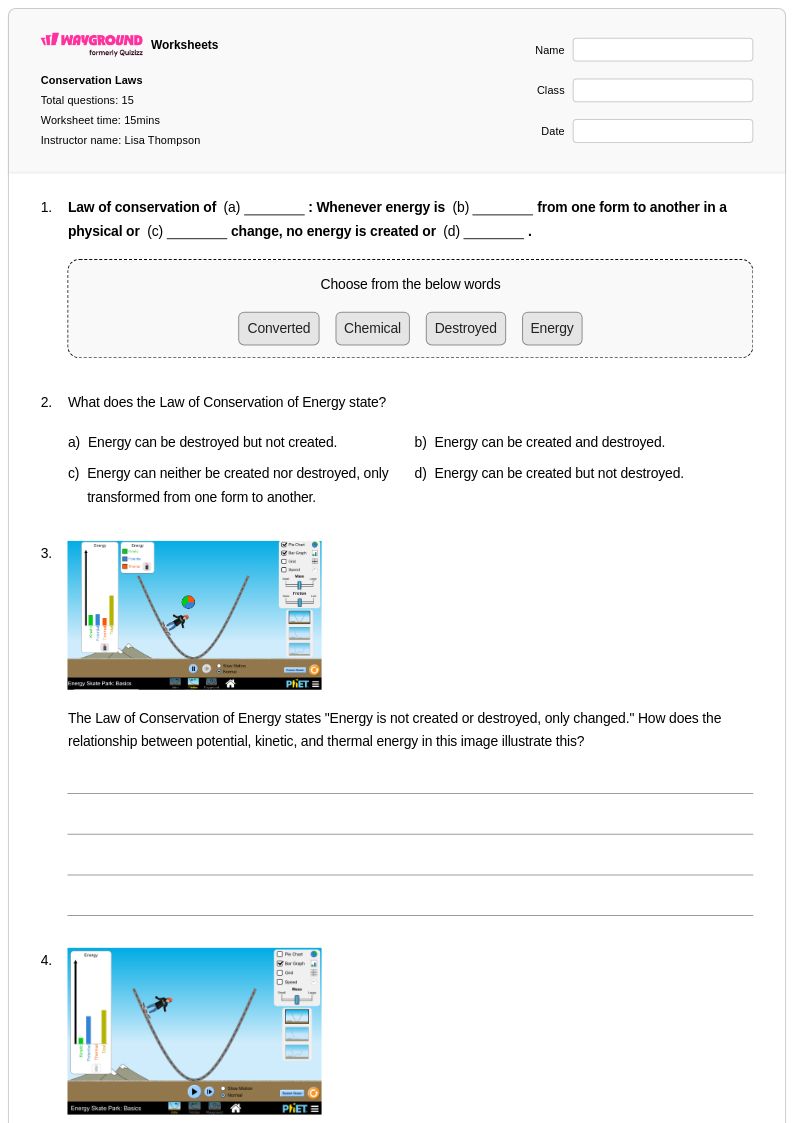
15 Q
8th - Uni

15 Q
8th - Uni

15 Q
9th

10 Q
9th

22 Q
8th - Uni

15 Q
6th - Uni

32 Q
9th

15 Q
9th
15 Q
8th - Uni

10 Q
9th - 12th
10 Q
9th - 12th
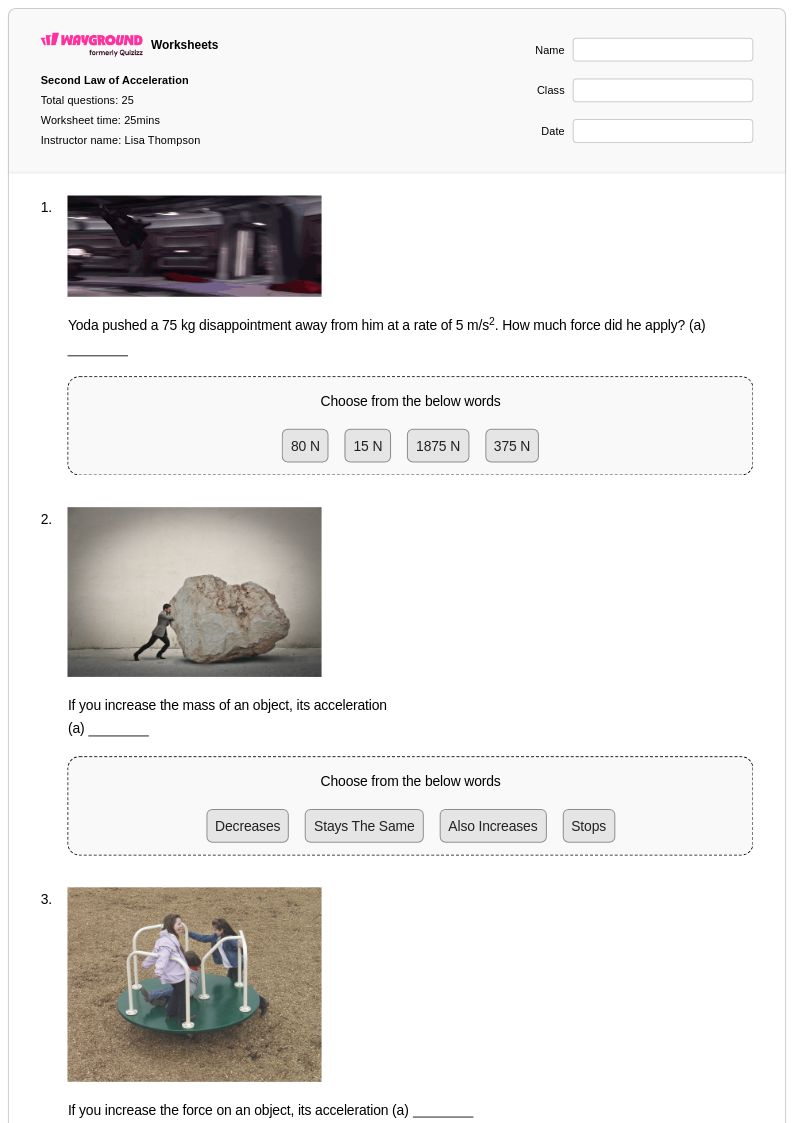
25 Q
8th - Uni

25 Q
9th - 12th
25 Q
8th - Uni
Explore Other Subject Worksheets for class 9
Explore printable Rate Laws worksheets for Class 9
Class 9 rate laws worksheets available through Wayground (formerly Quizizz) provide comprehensive practice with the mathematical relationships that govern chemical reaction speeds. These expertly designed resources strengthen students' abilities to determine reaction orders, calculate rate constants, and predict how changes in reactant concentrations affect reaction rates. The worksheets feature systematic practice problems that guide students through interpreting experimental data, constructing rate law expressions, and applying integrated rate equations for zero, first, and second-order reactions. Each printable collection includes detailed answer keys that support independent learning and self-assessment, while free pdf formats ensure accessibility for diverse classroom needs and study environments.
Wayground (formerly Quizizz) empowers chemistry teachers with millions of educator-created rate laws resources that streamline lesson planning and differentiated instruction. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific chemistry standards and tailored to Class 9 proficiency levels. Customization tools enable instructors to modify existing materials or combine multiple resources to address varying student needs, from foundational rate law concepts to advanced kinetics applications. These versatile materials are available in both digital and printable pdf formats, supporting flexible implementation for in-class practice, homework assignments, remediation sessions, and enrichment activities that deepen conceptual understanding of chemical kinetics principles.
