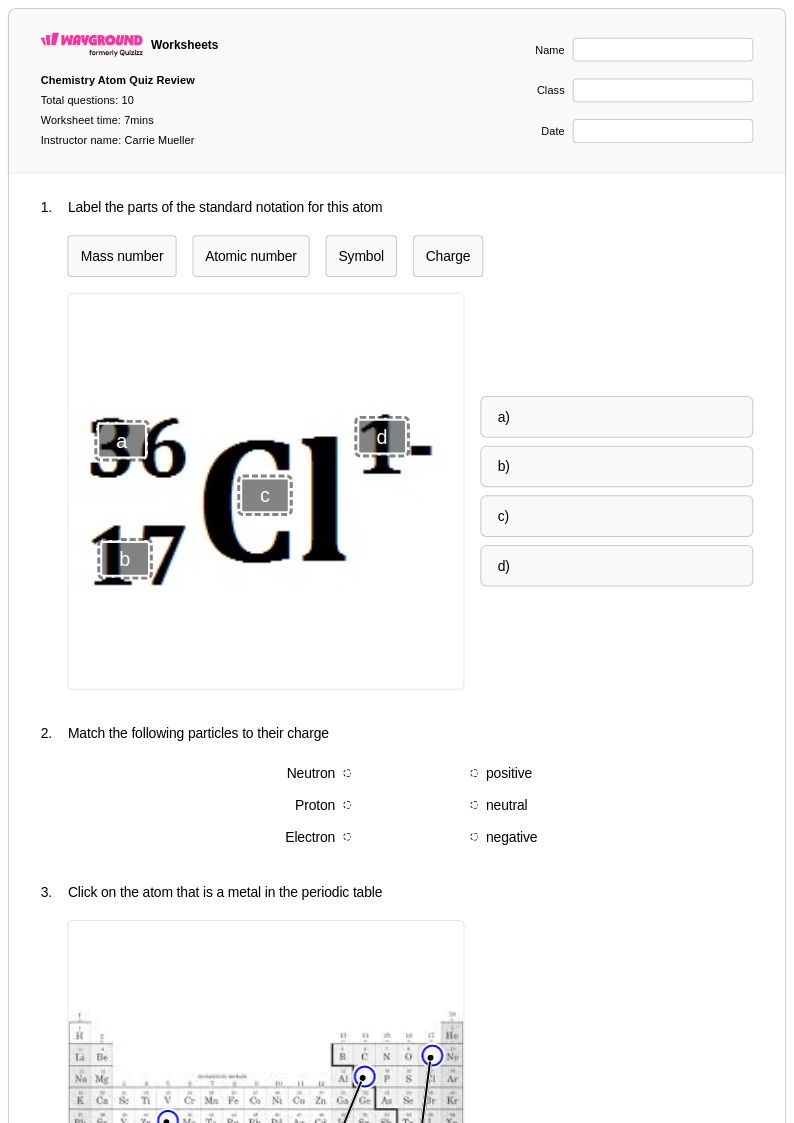
10 Q
10th

26 Q
8th
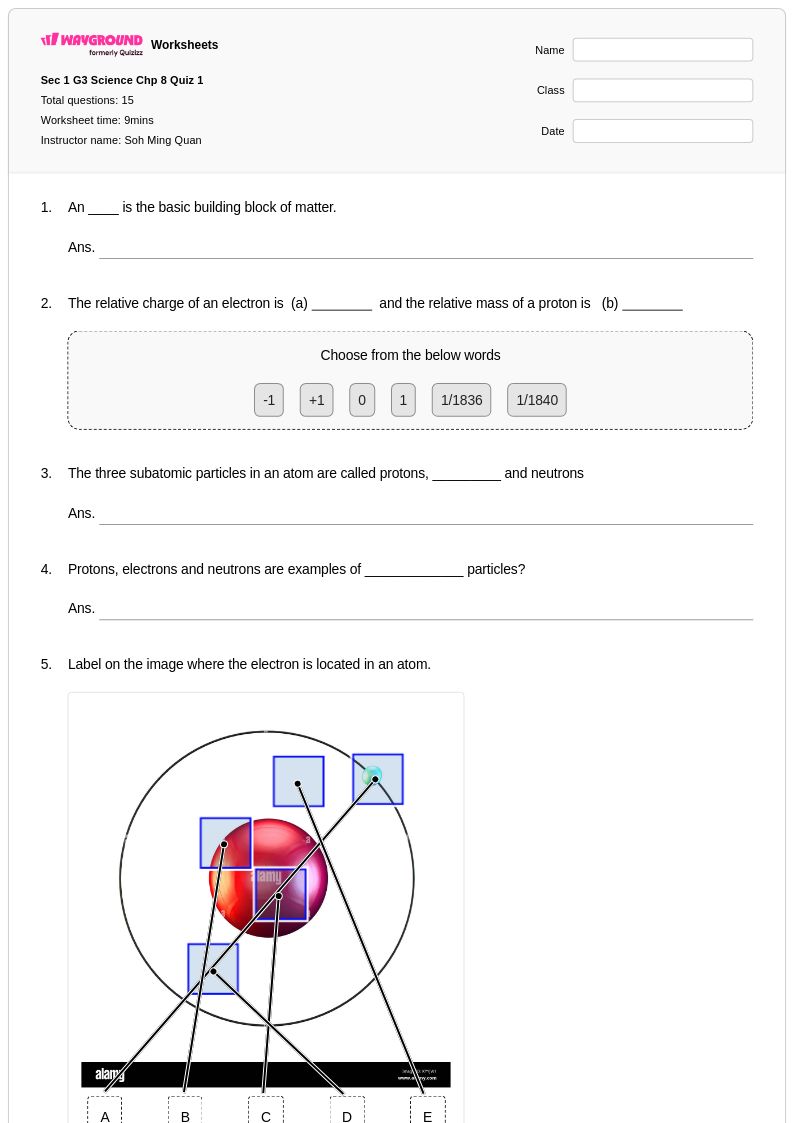
15 Q
7th
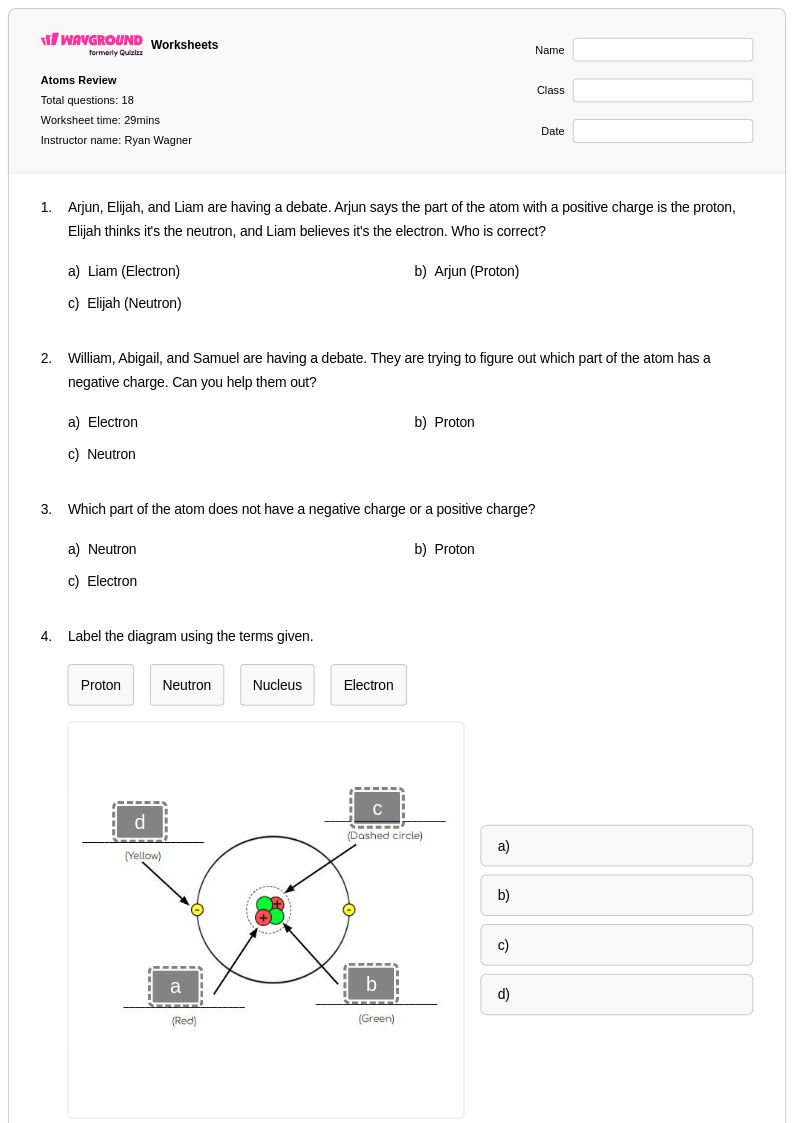
18 Q
6th

15 Q
10th
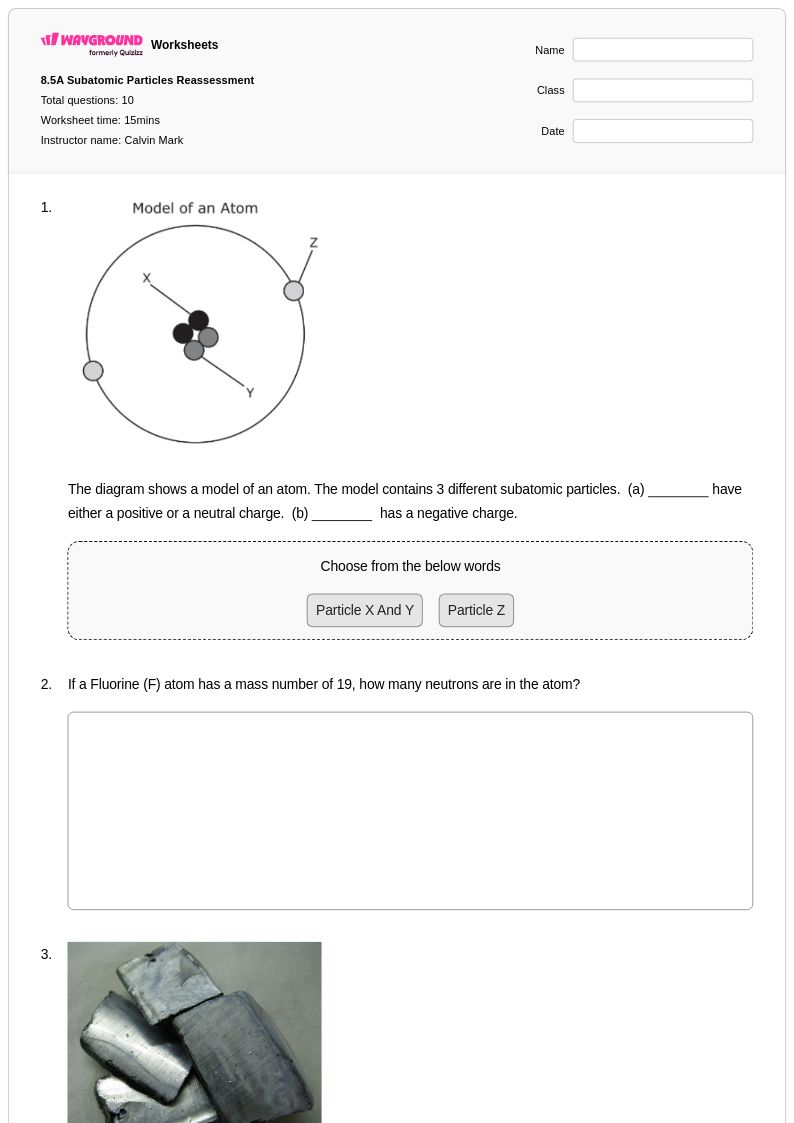
10 Q
8th
18 Q
10th
20 Q
8th

13 Q
7th

16 Q
9th
17 Q
9th
21 Q
6th

10 Q
8th
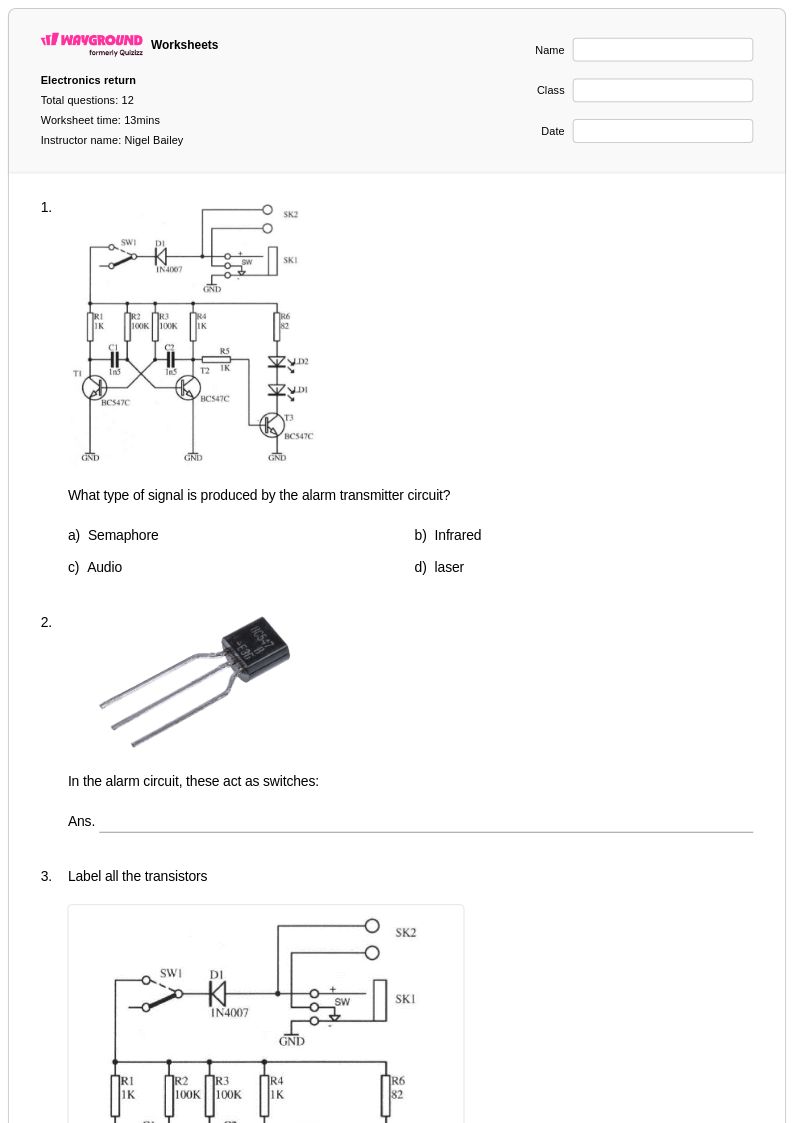
12 Q
PD
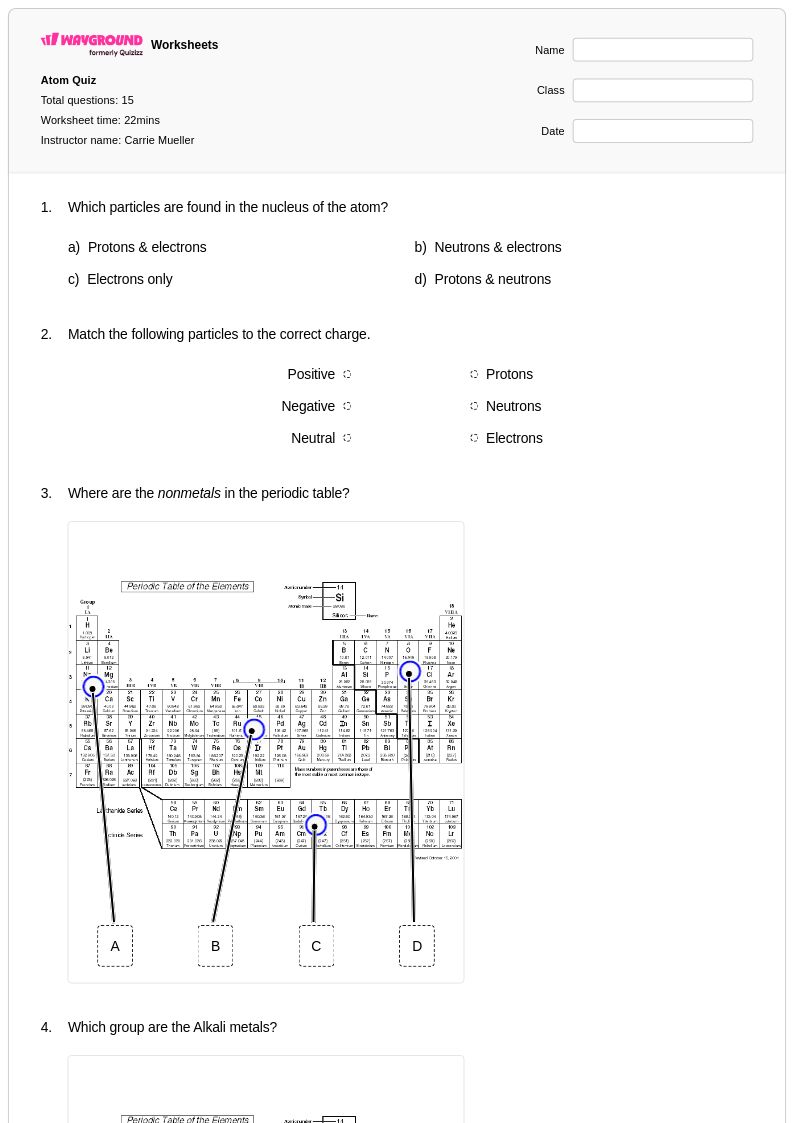
15 Q
10th

95 Q
6th - 8th
20 Q
3rd
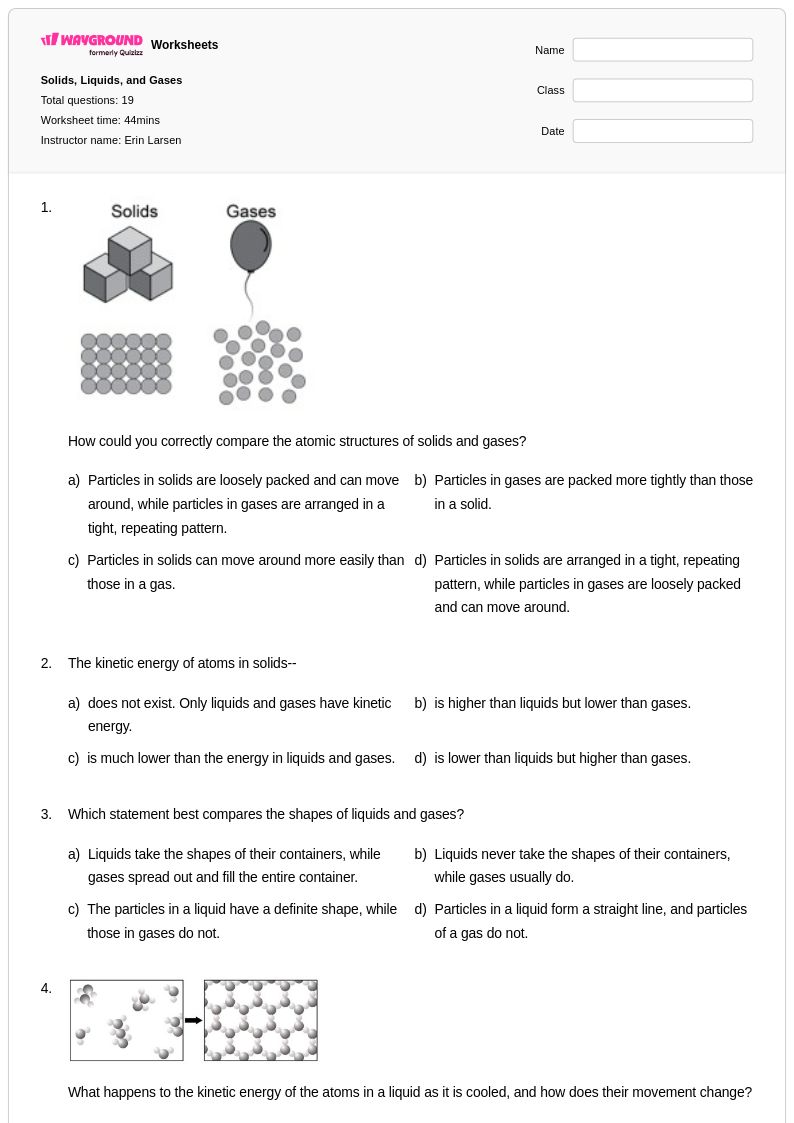
20 Q
6th
15 Q
6th
15 Q
8th

10 Q
6th
12 Q
9th - 12th

15 Q
6th - 8th

20 Q
8th
Explore Worksheets by Subjects
Explore printable Electronic Structure of Atoms worksheets
Electronic structure of atoms worksheets available through Wayground (formerly Quizizz) provide comprehensive coverage of fundamental atomic theory concepts that form the cornerstone of chemistry education. These carefully crafted resources help students master critical skills including electron configuration notation, orbital diagrams, quantum number assignments, and periodic trends related to atomic structure. The worksheets feature systematic practice problems that guide learners through complex topics such as aufbau principle applications, Hund's rule implementation, and the relationship between electron arrangements and chemical properties. Each resource includes detailed answer keys that support both independent study and classroom instruction, while the free printable pdf format ensures accessibility for diverse learning environments and study preferences.
Wayground's extensive collection draws from millions of teacher-created resources specifically designed to address the intricate concepts within electronic structure of atoms instruction. The platform's advanced search and filtering capabilities enable educators to quickly locate materials aligned with specific curriculum standards and learning objectives, while built-in differentiation tools allow seamless customization for varying student ability levels. Teachers can efficiently modify worksheets to support remediation for struggling learners or provide enrichment challenges for advanced students, with both printable and digital pdf formats offering maximum flexibility for classroom implementation. This comprehensive approach to resource management streamlines lesson planning while ensuring that students receive targeted skill practice in essential areas such as electron shell filling, valence electron identification, and the connection between atomic structure and chemical behavior.
