
15 Q
10th - Uni
15 Q
10th - Uni

15 Q
10th - Uni

25 Q
10th - Uni

50 Q
10th
25 Q
9th - 12th

13 Q
10th

15 Q
12th - Uni

40 Q
10th

24 Q
12th

21 Q
7th

30 Q
7th

20 Q
7th

13 Q
7th

26 Q
9th

20 Q
10th
85 Q
12th - Uni

20 Q
7th
23 Q
7th
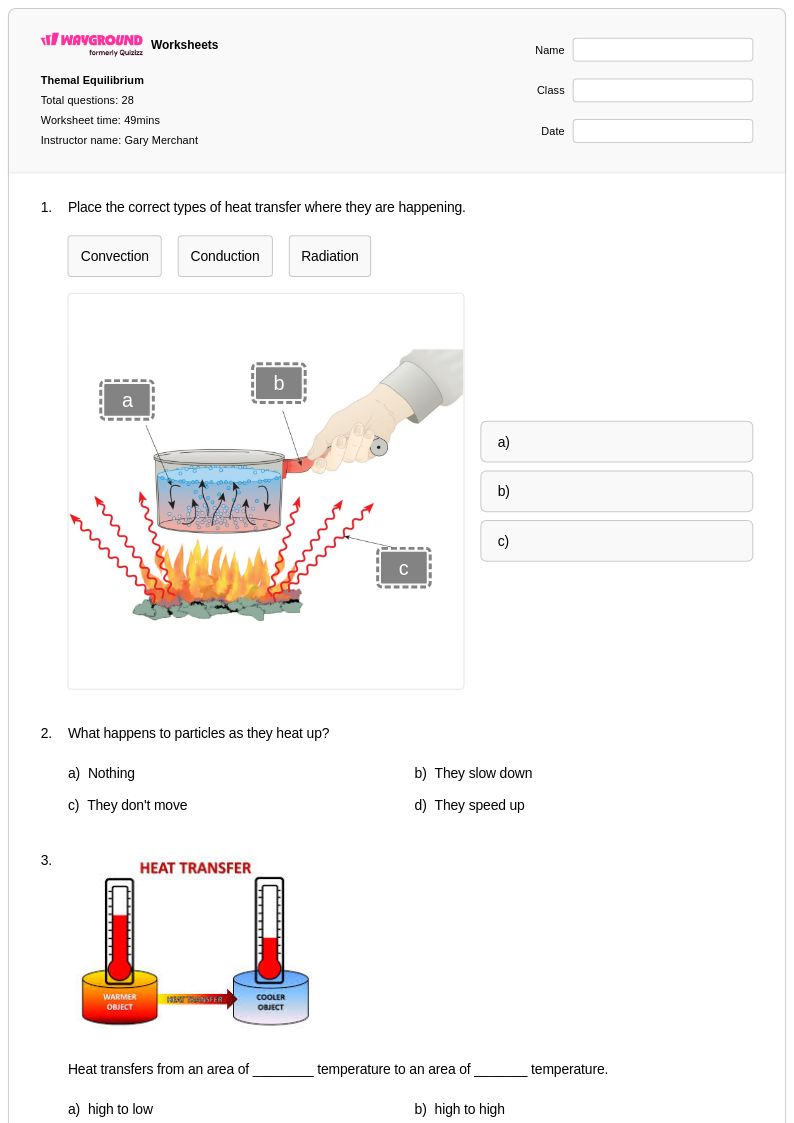
30 Q
7th

24 Q
9th
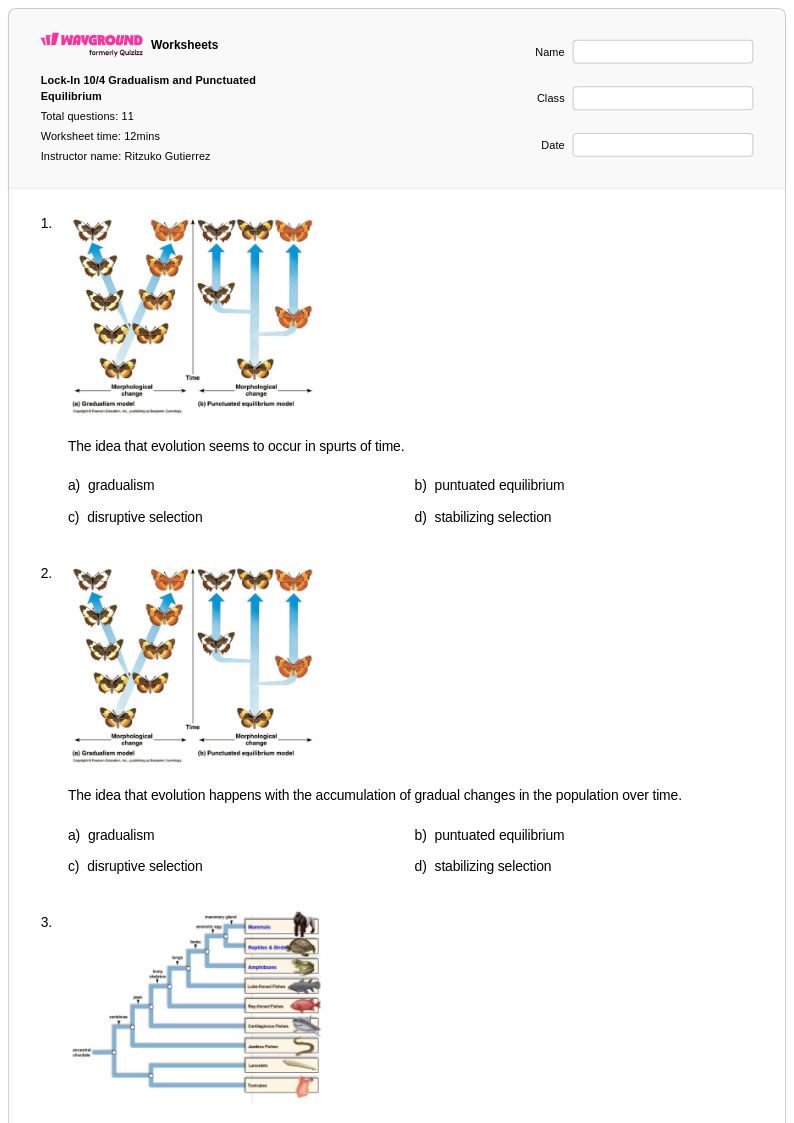
11 Q
12th
21 Q
5th
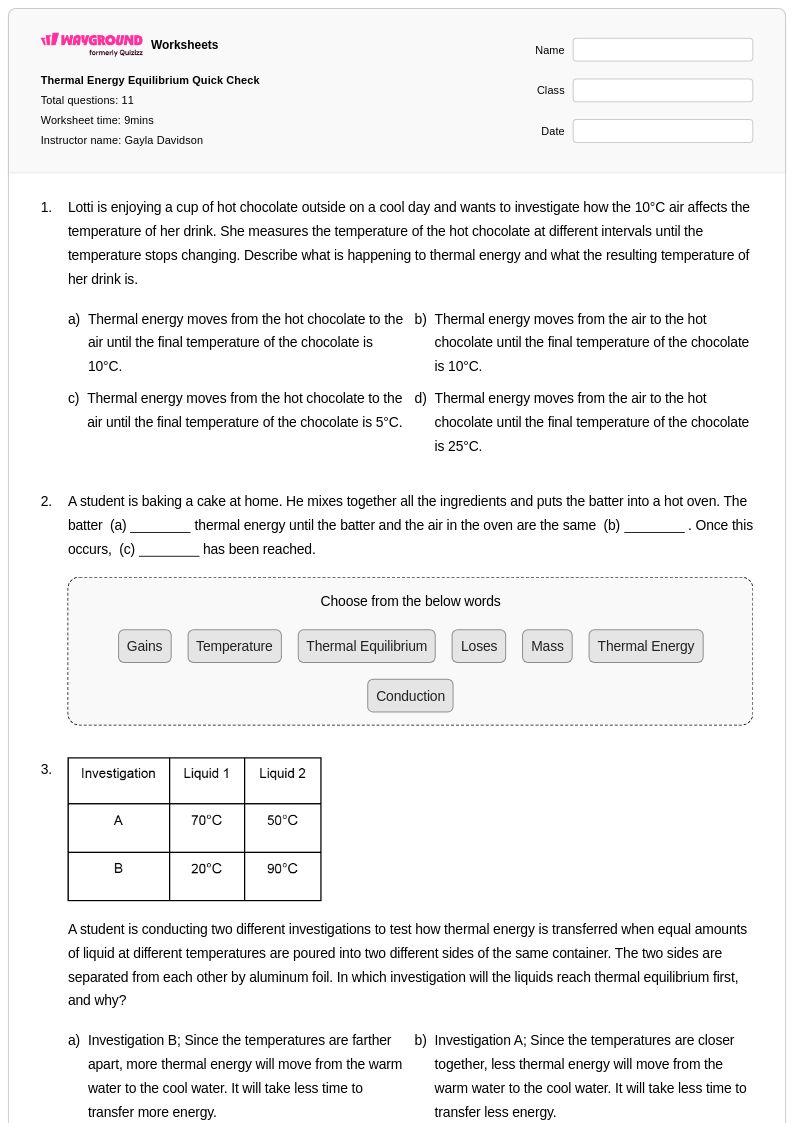
11 Q
7th
Explore Worksheets by Subjects
Explore printable Equilibrium Expressions worksheets
Equilibrium expressions represent a fundamental concept in chemical equilibrium, requiring students to master the mathematical relationships that describe reaction dynamics at equilibrium. Wayground's comprehensive collection of equilibrium expressions worksheets provides targeted practice with writing and interpreting equilibrium constant expressions, calculating Kc and Kp values, and understanding the relationship between reaction quotients and equilibrium constants. These carefully designed practice problems strengthen students' ability to analyze chemical systems, apply Le Chatelier's principle through quantitative analysis, and solve complex equilibrium calculations. Each worksheet includes a complete answer key and is available as a free printable pdf, allowing students to work through progressively challenging scenarios that build confidence in manipulating equilibrium expressions and interpreting their chemical significance.
Wayground's platform, formerly known as Quizizz, empowers educators with access to millions of teacher-created resources specifically focused on equilibrium expressions and related chemical equilibrium concepts. The platform's advanced search and filtering capabilities enable teachers to locate worksheets that align with specific curriculum standards and match their students' skill levels, while built-in differentiation tools support both remediation for struggling learners and enrichment opportunities for advanced students. Teachers can customize existing worksheets or create entirely new practice sets, with all materials available in both printable pdf format and interactive digital versions. This flexibility streamlines lesson planning while providing educators with the resources needed to deliver targeted skill practice, assess student understanding of equilibrium calculations, and ensure mastery of these critical chemical principles through repeated, varied exposure to equilibrium expression problems.
