
8 Q
9th
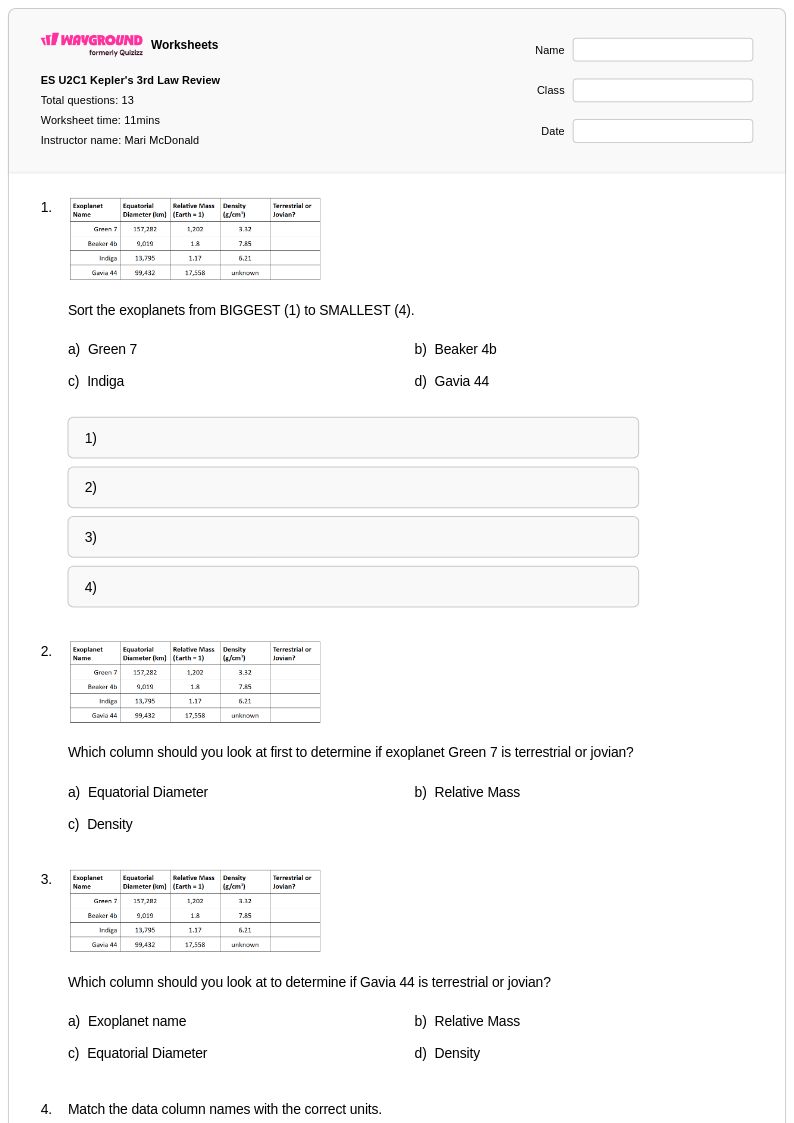
13 Q
9th - 12th

20 Q
9th

24 Q
9th

25 Q
9th
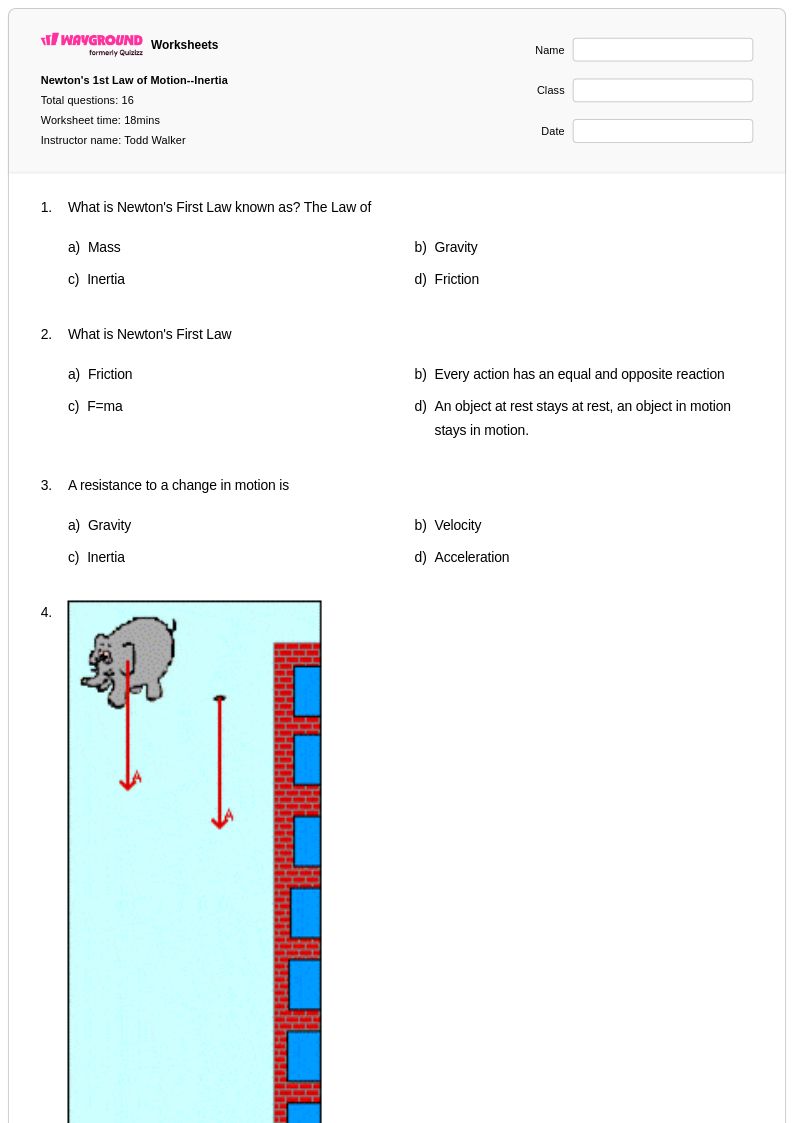
16 Q
9th - 12th

20 Q
8th - 9th

31 Q
9th - 12th
13 Q
9th
14 Q
8th - 10th

22 Q
9th

15 Q
7th - Uni

21 Q
8th - Uni

15 Q
9th - 12th
14 Q
9th - 12th

10 Q
9th - 12th

32 Q
9th

15 Q
9th

10 Q
9th - 12th
47 Q
8th - Uni

20 Q
9th - 12th

20 Q
9th - 12th
55 Q
9th - 12th

18 Q
9th - 12th
Explore Other Subject Worksheets for year 9
Explore printable Dalton's Law worksheets for Year 9
Dalton's Law worksheets for Year 9 students provide comprehensive practice with partial pressure calculations and gas mixture analysis, fundamental concepts in chemistry that bridge theoretical understanding with practical applications. These carefully designed resources help students master the principle that total pressure in a gas mixture equals the sum of individual partial pressures, strengthening their problem-solving abilities through systematic practice problems that range from basic conceptual questions to complex multi-step calculations. The worksheets include detailed answer keys that guide students through proper solution methods, while the free printable pdf format ensures accessibility for both classroom instruction and independent study, allowing educators to seamlessly integrate these materials into their existing curriculum structure.
Wayground, formerly Quizizz, empowers chemistry teachers with an extensive collection of millions of teacher-created Dalton's Law resources that streamline lesson planning and enhance student learning outcomes. The platform's robust search and filtering capabilities allow educators to quickly locate worksheets aligned with specific standards and learning objectives, while built-in differentiation tools enable customization for varying skill levels within Year 9 classrooms. Teachers can access these materials in both printable and digital pdf formats, facilitating flexible implementation whether for whole-class instruction, targeted remediation for struggling students, or enrichment activities for advanced learners, ultimately supporting diverse pedagogical approaches while maintaining consistent focus on essential partial pressure concepts and gas law applications.
