20 Q
9th - 12th

22 Q
9th - 12th

14 Q
9th - 12th
10 Q
12th
30 Q
10th - Uni

40 Q
12th

20 Q
9th - 12th

31 Q
12th

20 Q
9th - 12th
13 Q
9th - 12th

16 Q
9th - 12th
15 Q
12th

78 Q
12th
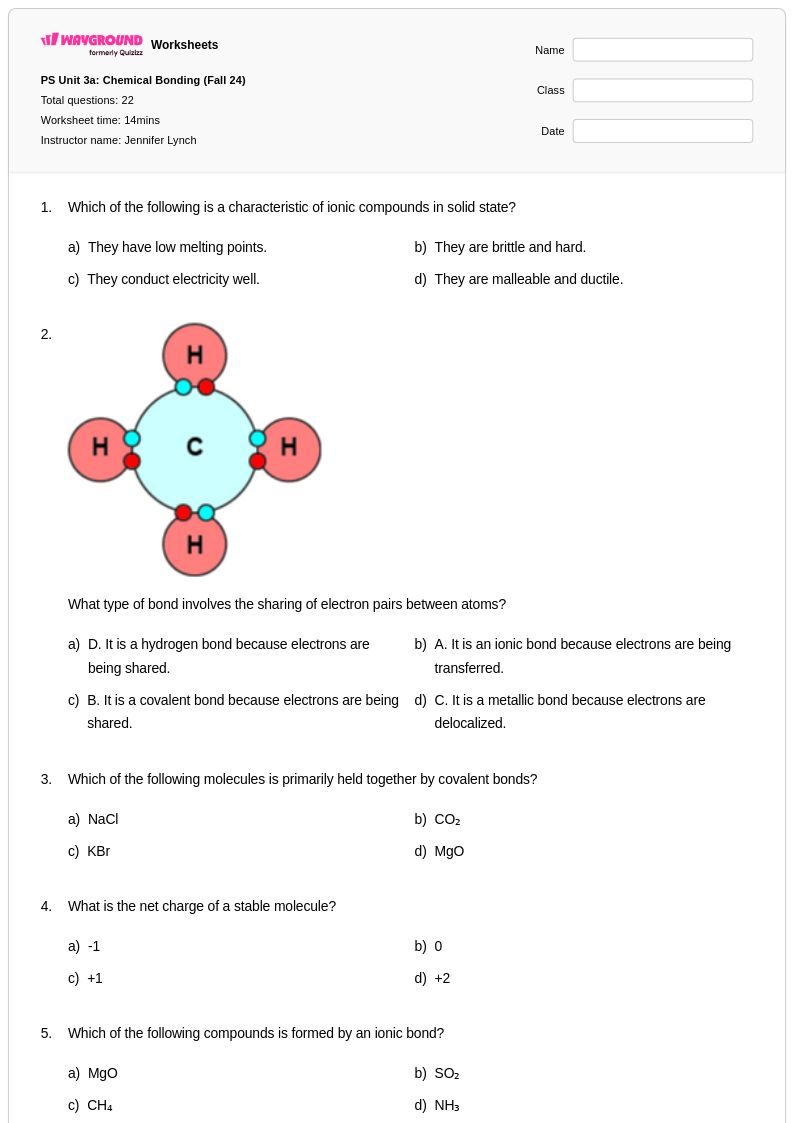
22 Q
9th - Uni

51 Q
9th - 12th
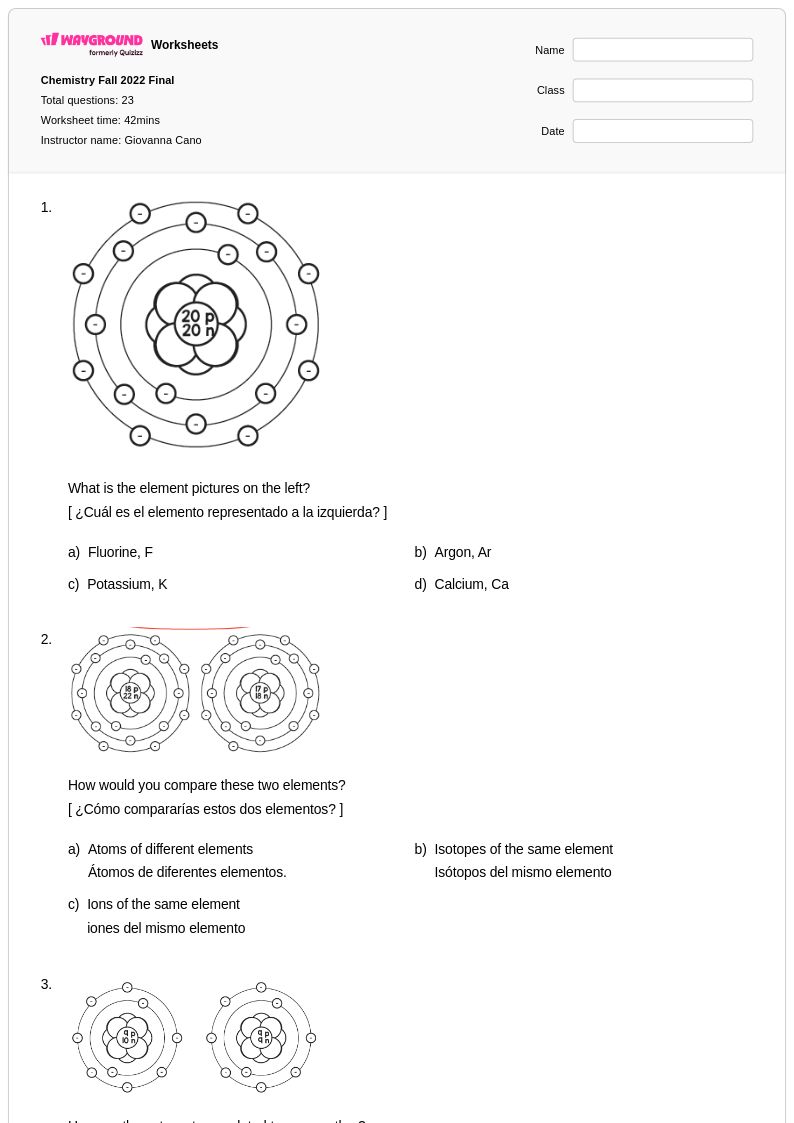
23 Q
12th

40 Q
9th - 12th

37 Q
11th - Uni
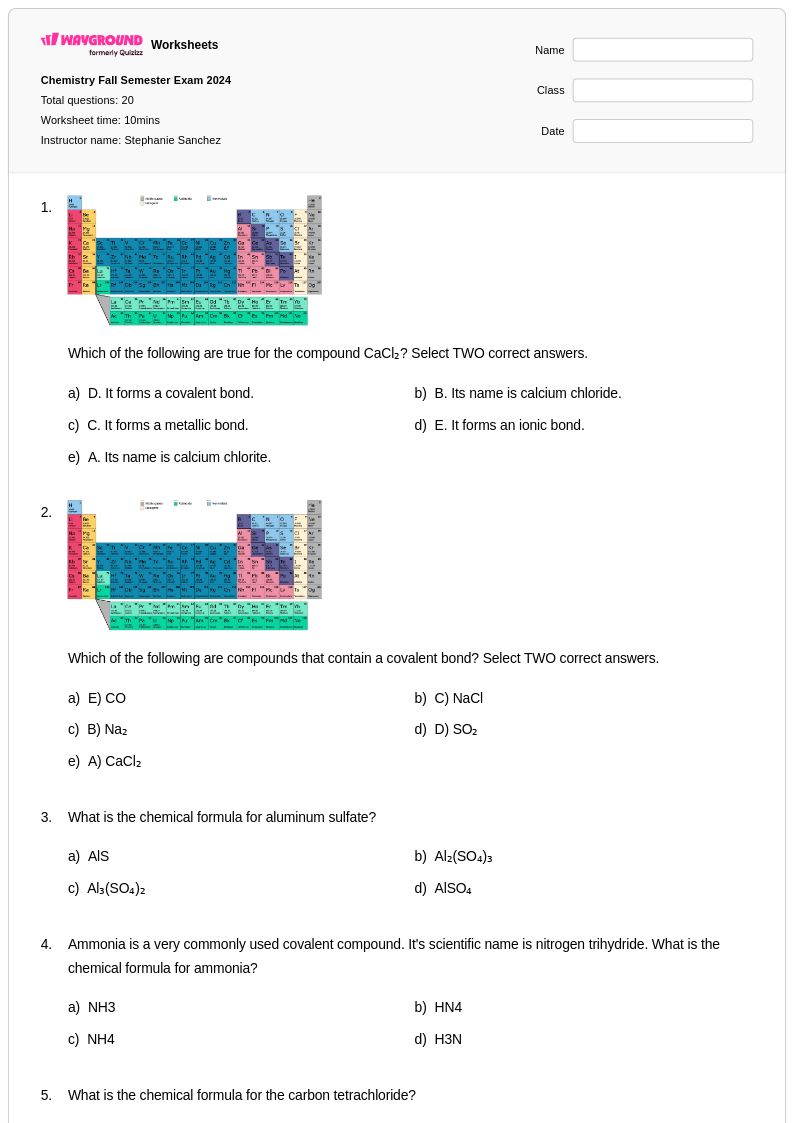
20 Q
11th - Uni

21 Q
9th - 12th
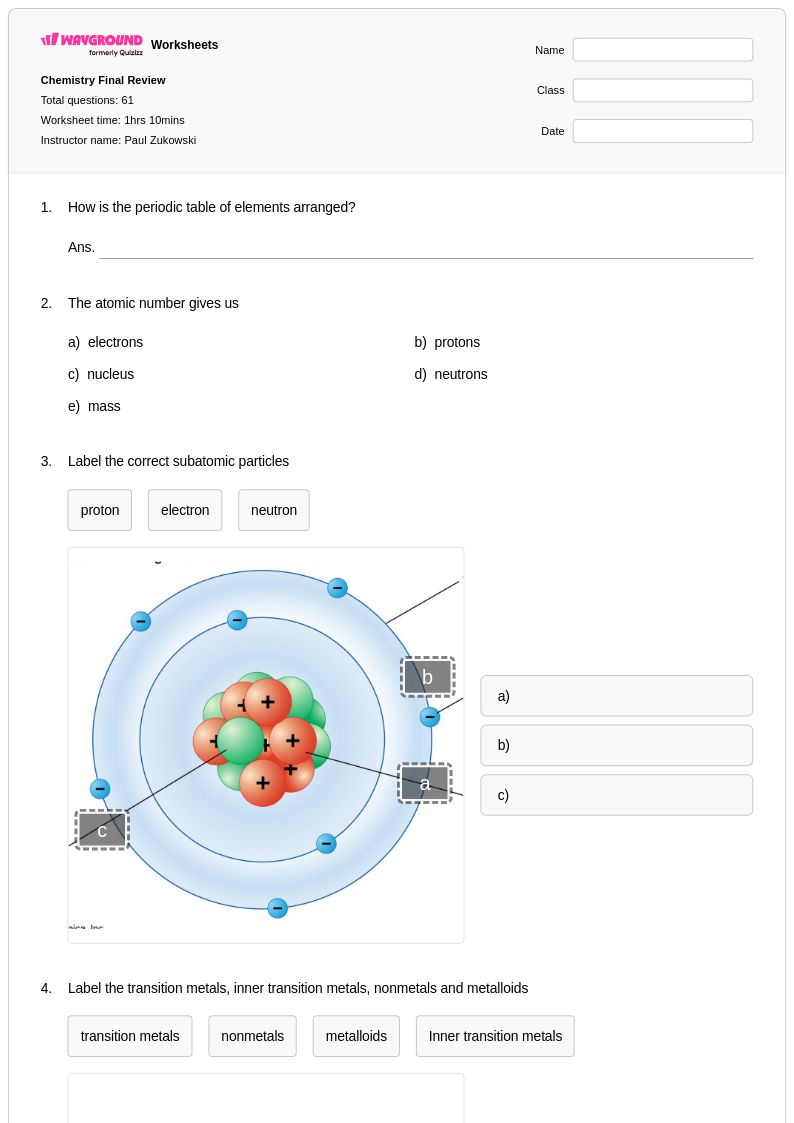
61 Q
12th
80 Q
9th - 12th
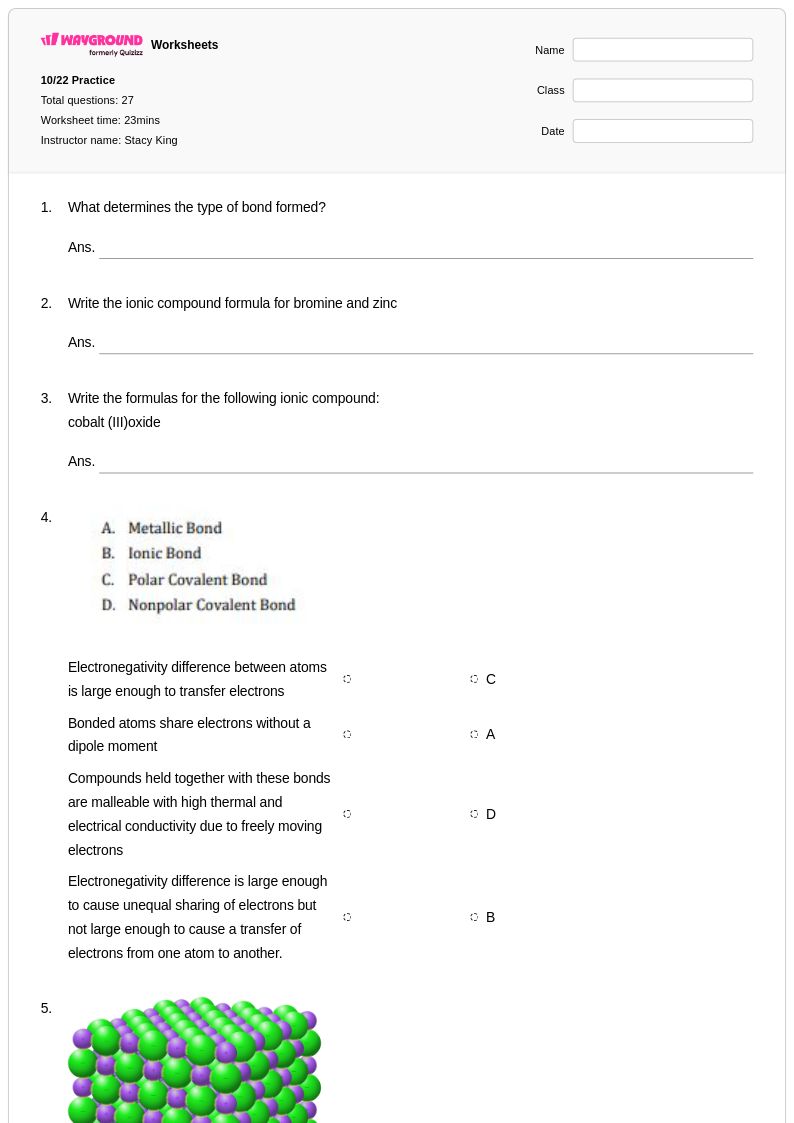
27 Q
9th - 12th
72 Q
12th
Explore Other Subject Worksheets for grade 12
Explore printable Ionic Formulas worksheets for Grade 12
Ionic formulas represent a fundamental concept in Grade 12 chemistry that requires students to master the systematic approach to writing chemical formulas for ionic compounds. Wayground's comprehensive collection of ionic formulas worksheets provides students with extensive practice in determining the correct ratios of cations and anions, applying oxidation states, and writing balanced ionic equations. These carefully designed practice problems strengthen critical skills including identifying polyatomic ions, calculating formula units, and predicting ionic compound formation based on electron transfer principles. The worksheets include detailed answer keys that guide students through step-by-step solutions, while the free printable pdf format ensures accessibility for both classroom instruction and independent study sessions.
Wayground, formerly Quizizz, empowers chemistry educators with millions of teacher-created resources specifically targeting ionic formulas and related chemical concepts, all searchable through advanced filtering options that align with state and national chemistry standards. Teachers can easily customize these worksheet collections to match their specific curriculum needs, differentiating content for students who require additional remediation or advanced enrichment opportunities. The platform's flexible digital and printable formats, including downloadable pdf versions, streamline lesson planning while providing versatile options for both traditional classroom settings and remote learning environments. These comprehensive tools support effective skill practice through varied problem types, from basic binary ionic compounds to complex polyatomic ion combinations, ensuring students develop the analytical thinking required for advanced chemistry coursework.
