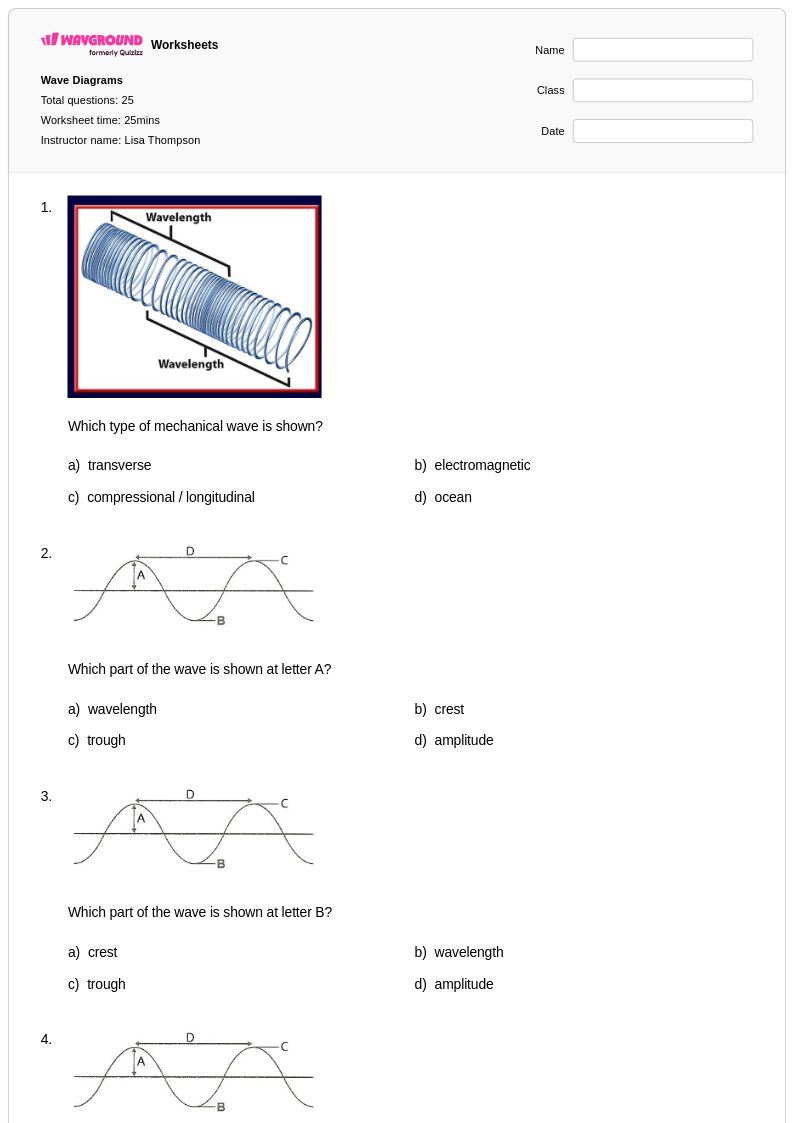
25Q
8th - Uni
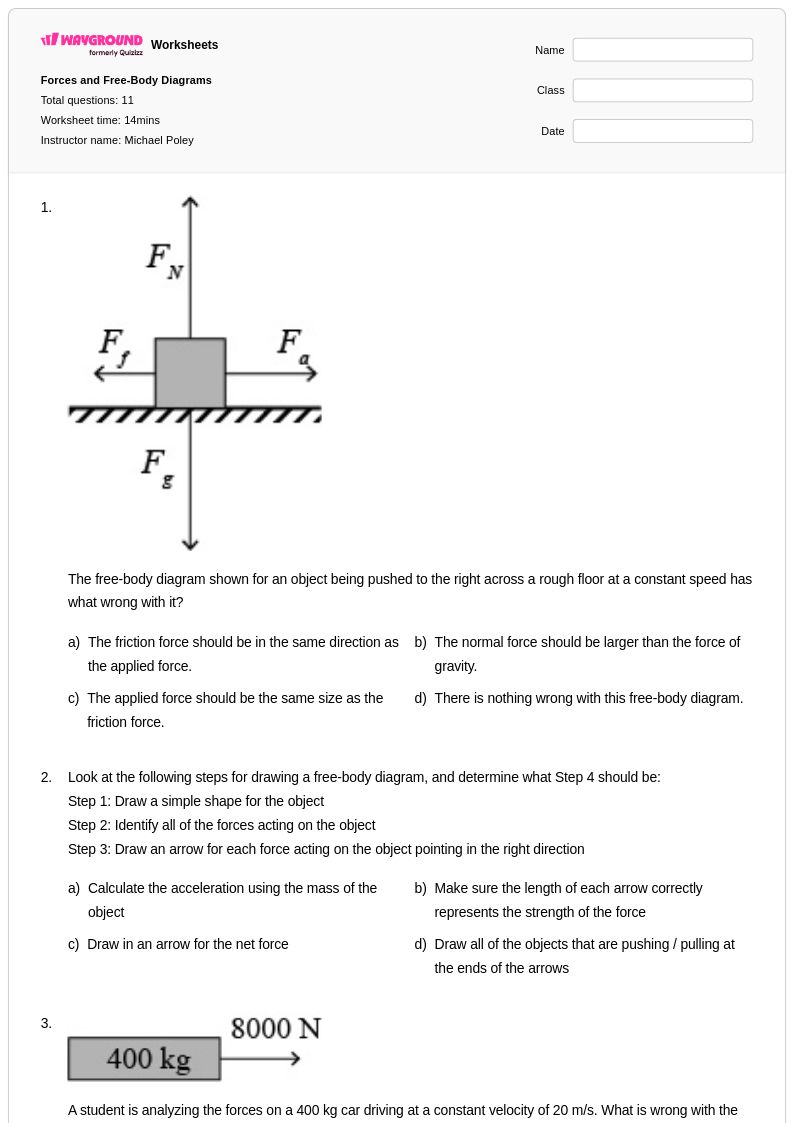
11Q
9th - 12th
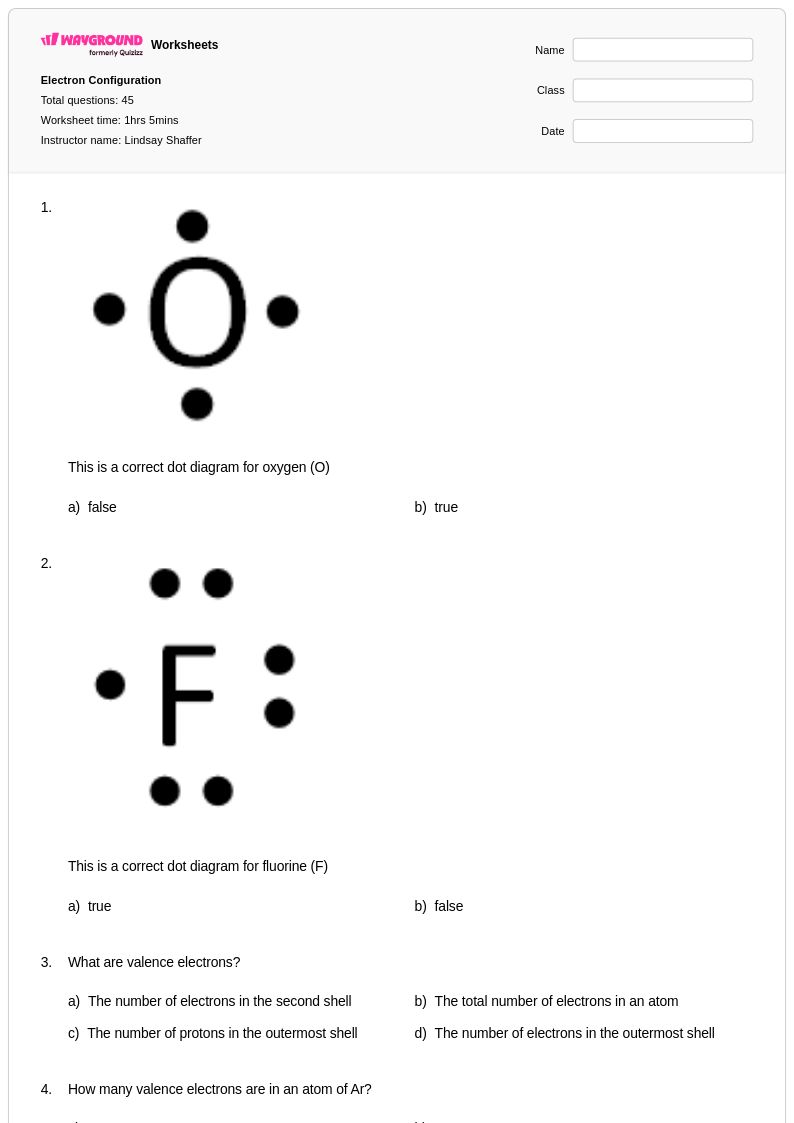
45Q
11th

222Q
11th
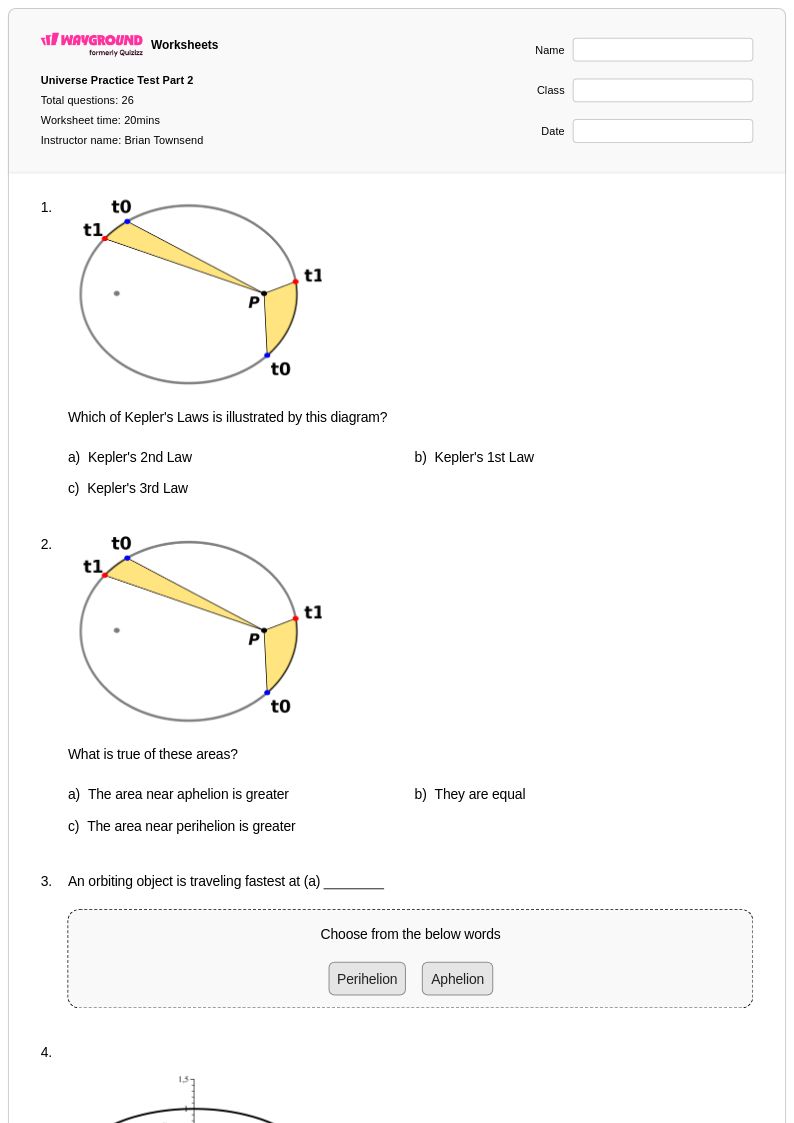
26Q
11th

6Q
11th
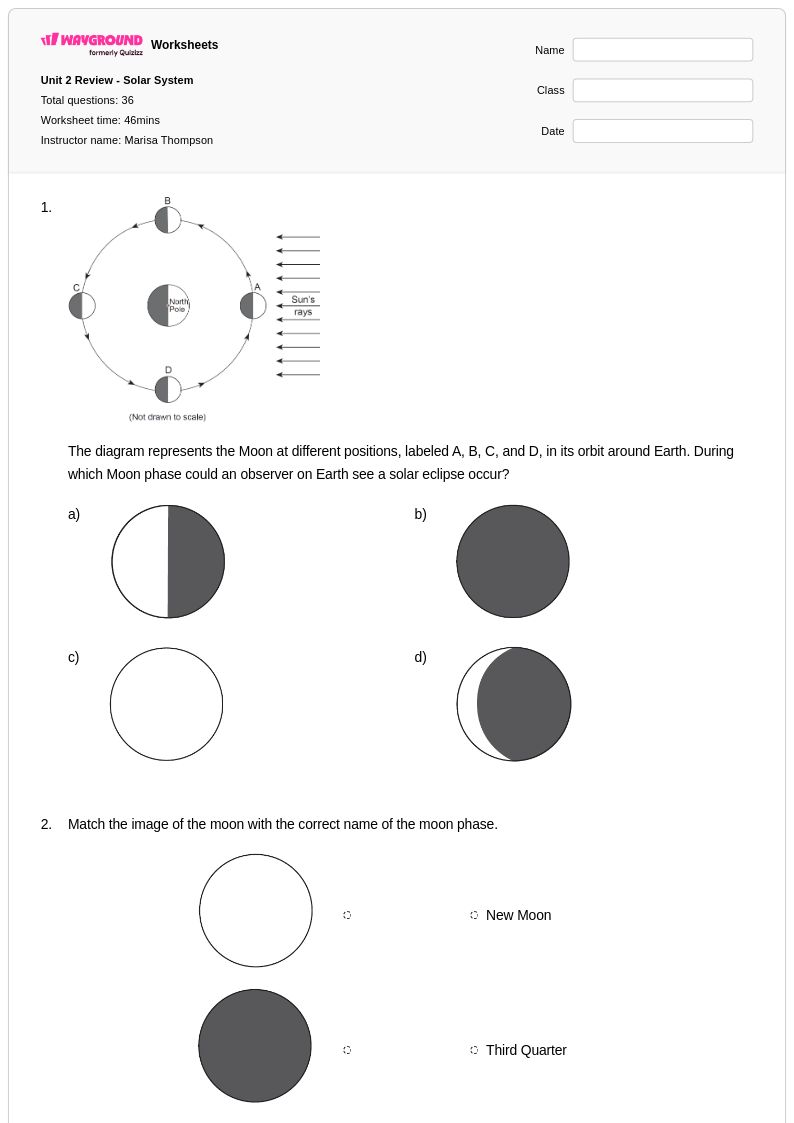
36Q
9th - 12th
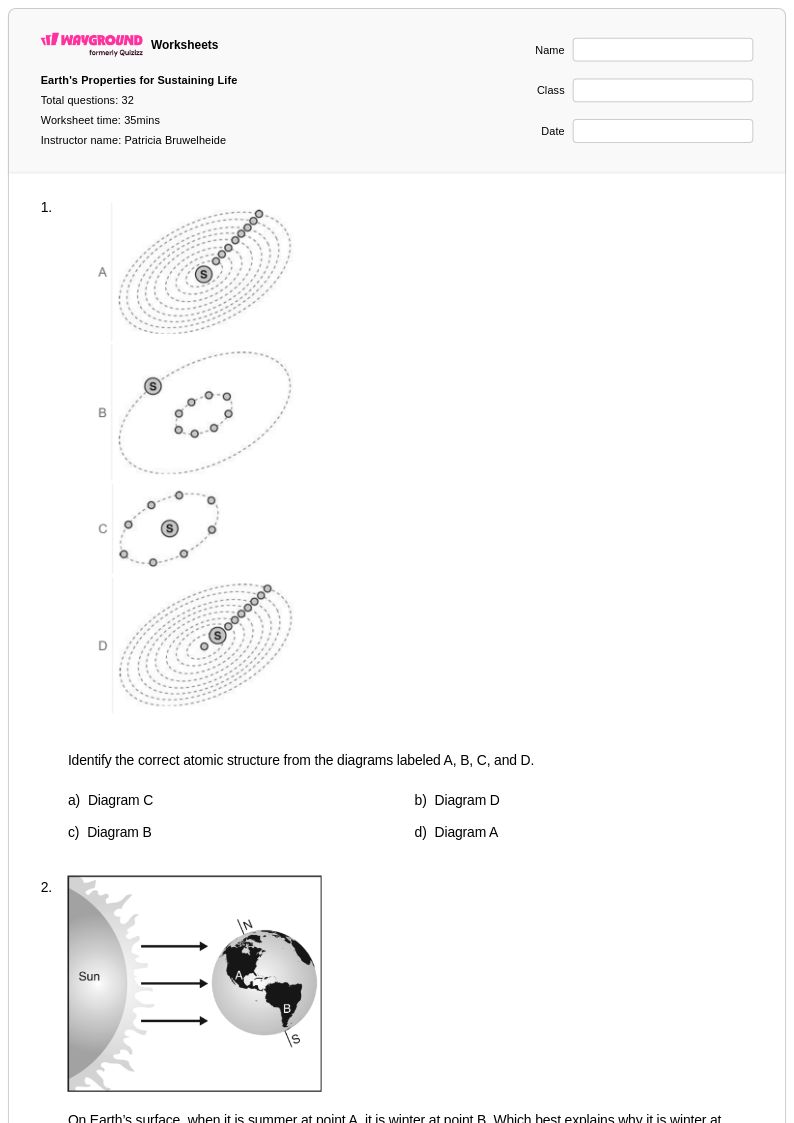
32Q
6th - Uni

50Q
9th - 12th
30Q
9th - 12th

20Q
9th - 12th

6Q
11th

10Q
9th - 12th

20Q
11th
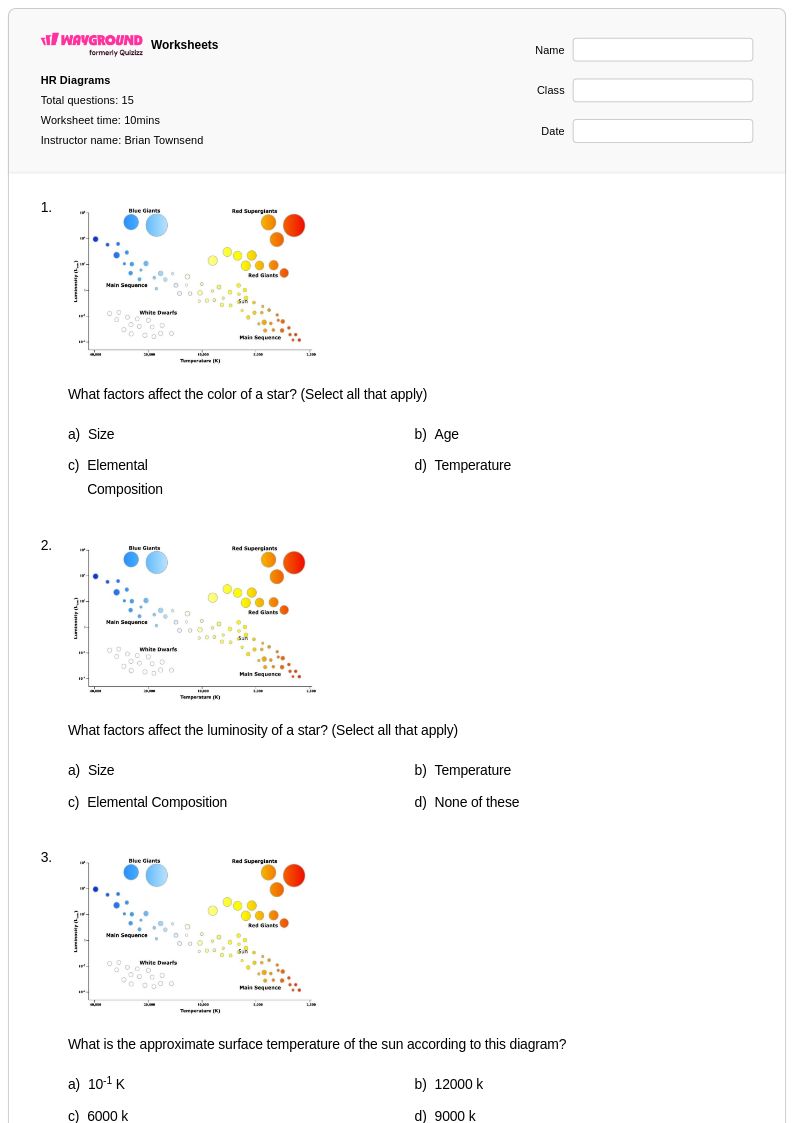
15Q
11th
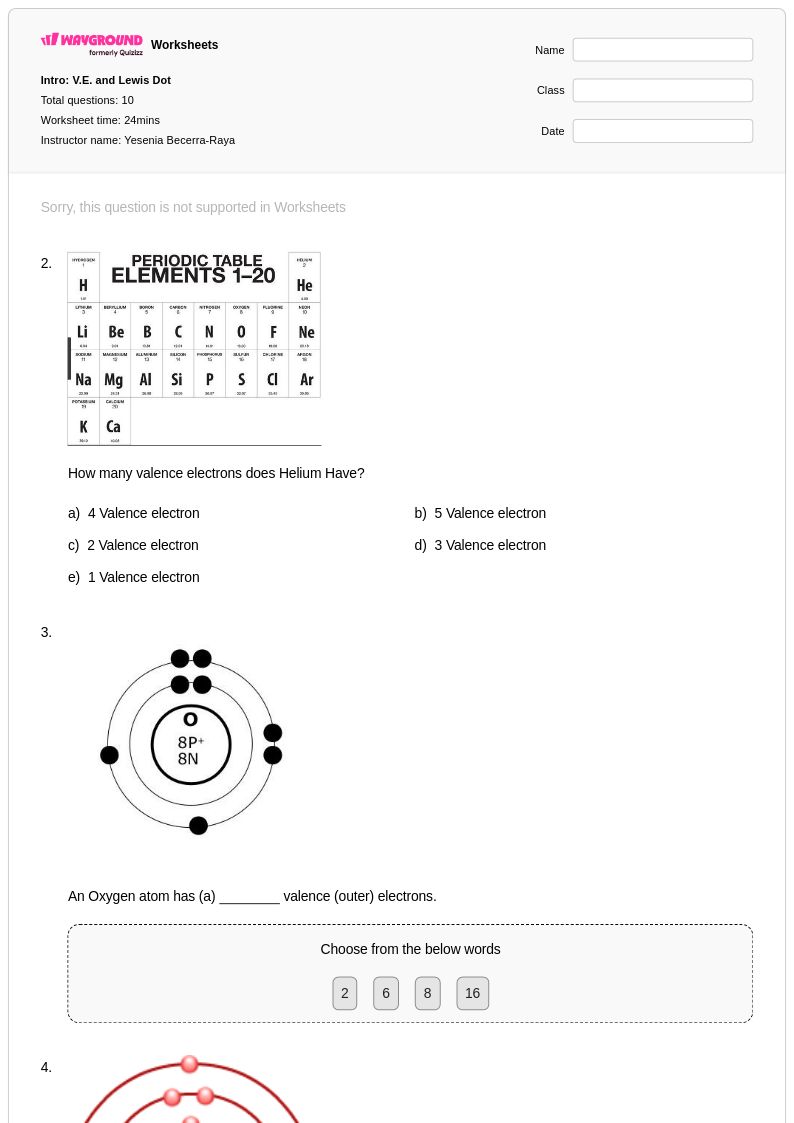
25Q
9th - 12th
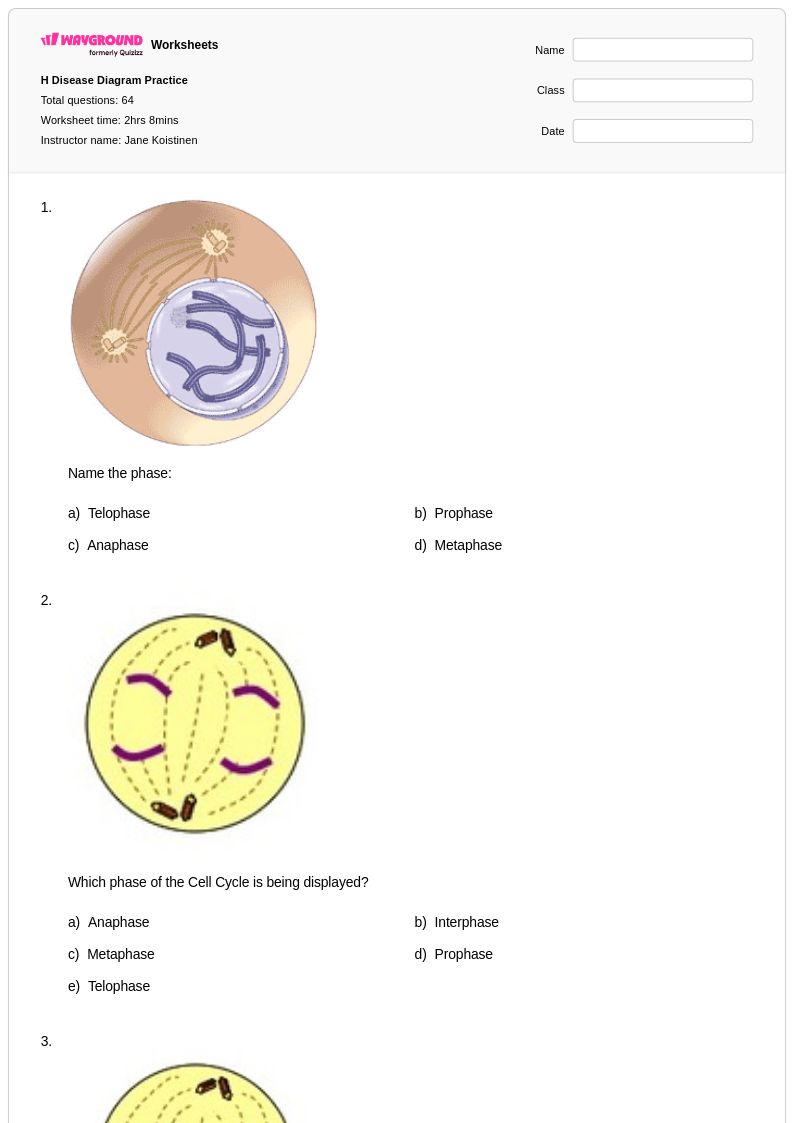
64Q
9th - 12th
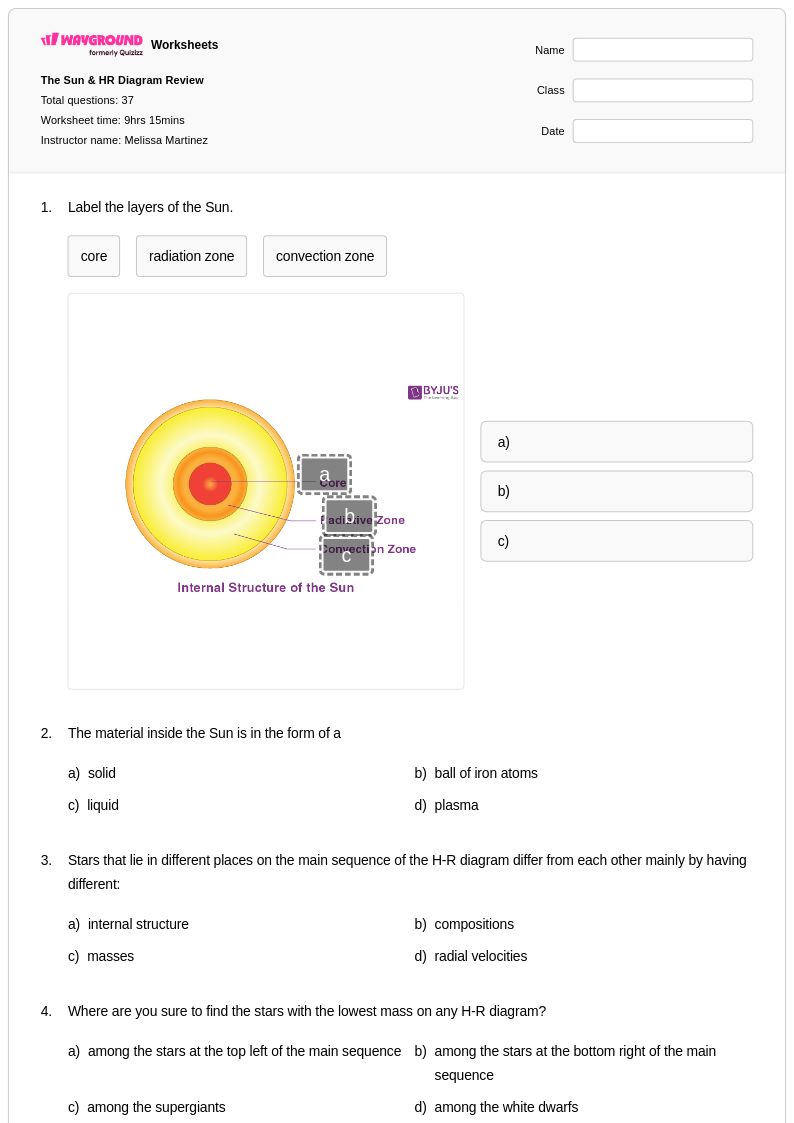
37Q
9th - 12th

16Q
5th - Uni

20Q
8th - 12th
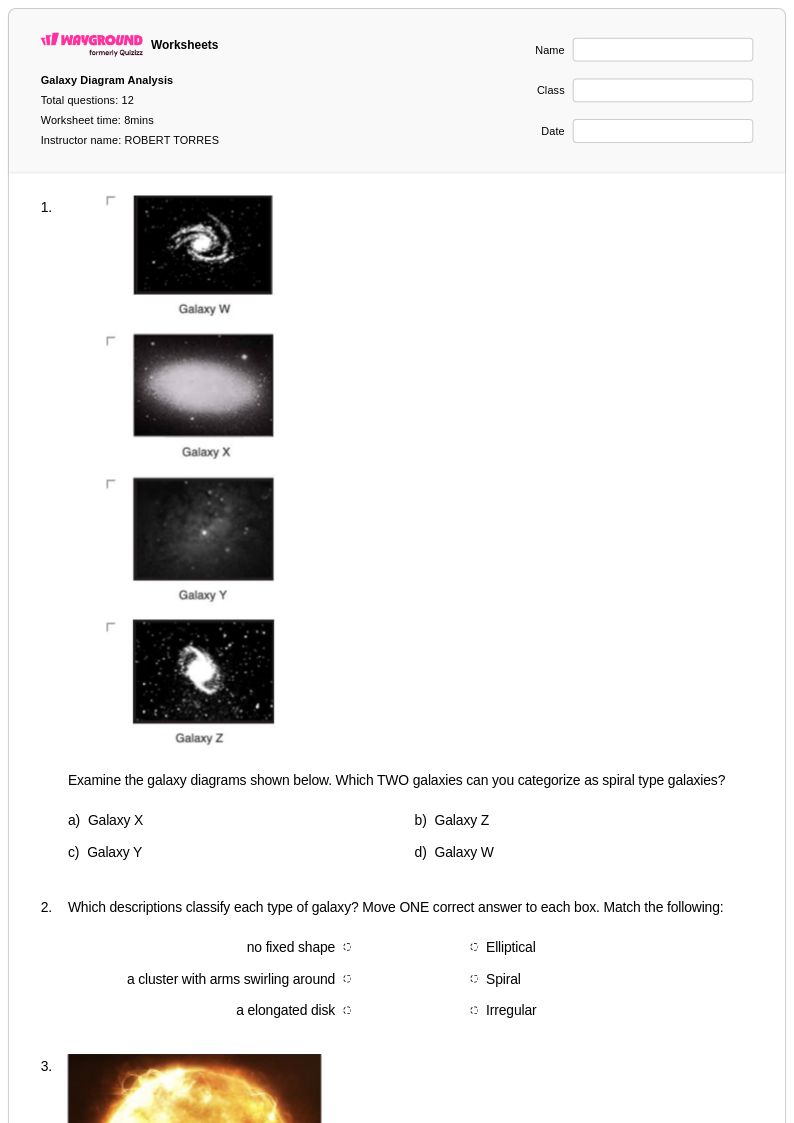
12Q
8th - Uni

21Q
11th

55Q
9th - 12th

16Q
9th - 12th
Explore otras hojas de trabajo de materias para grade 11
Explore printable Orbital Diagrams worksheets for Grade 11
Orbital diagrams represent a fundamental concept in Grade 11 chemistry that helps students visualize electron arrangements within atoms and understand the relationship between electron configuration and chemical behavior. Wayground's comprehensive collection of orbital diagram worksheets provides students with structured practice problems that develop their ability to construct accurate orbital diagrams using Hund's rule, the Aufbau principle, and Pauli exclusion principle. These free printables include detailed answer keys that allow students to self-check their work and identify common misconceptions about electron spin pairing and orbital filling order. The worksheets progress systematically from simple s and p orbital diagrams to more complex d and f orbital arrangements, strengthening students' foundational understanding of atomic structure and preparing them for advanced topics in chemical bonding and molecular geometry.
Wayground supports chemistry educators with millions of teacher-created orbital diagram resources that can be easily searched, filtered, and customized to meet diverse classroom needs. The platform's robust collection includes both printable pdf worksheets and interactive digital formats, allowing teachers to differentiate instruction based on student readiness levels and learning preferences. Standards-aligned content ensures that orbital diagram practice aligns with curriculum expectations, while flexible customization tools enable educators to modify existing worksheets or create new ones tailored to specific learning objectives. These resources prove invaluable for lesson planning, targeted remediation of electron configuration concepts, enrichment activities for advanced learners, and regular skill practice that reinforces the spatial reasoning and systematic thinking required to master orbital theory in chemistry.
