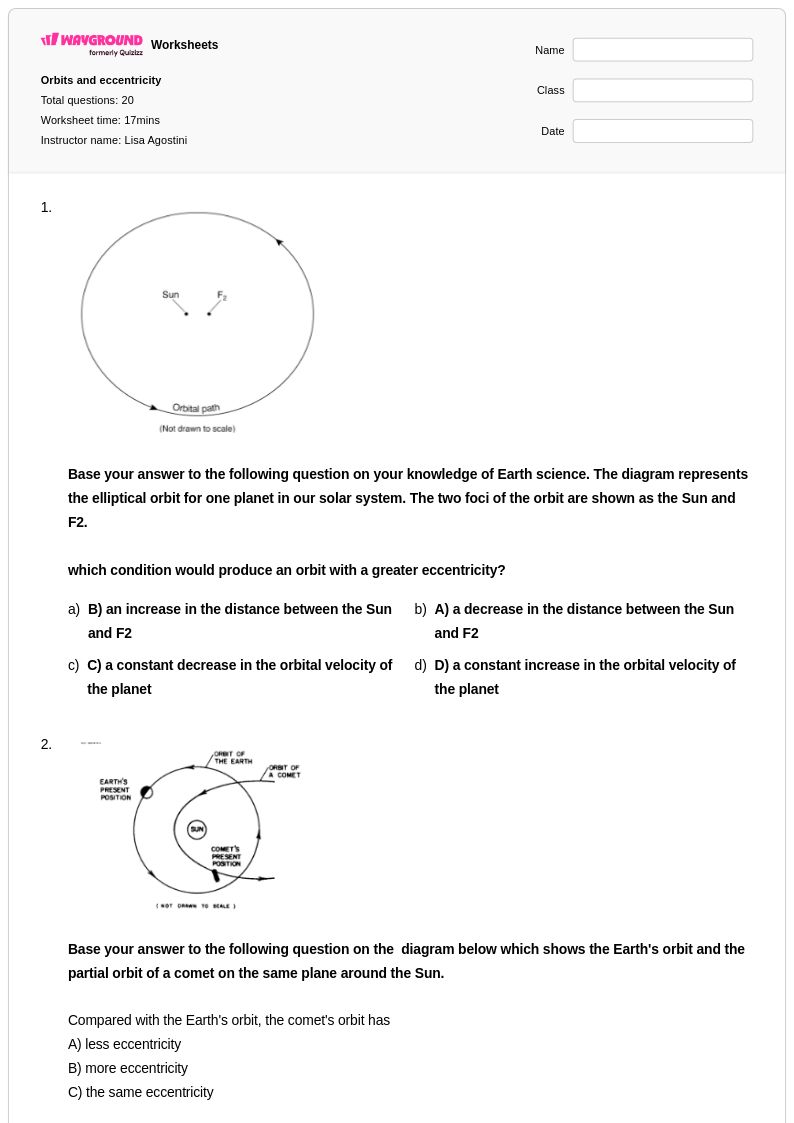
20 Q
9th - 12th

25 Q
8th - Uni

25 Q
8th - Uni
15 Q
8th - Uni
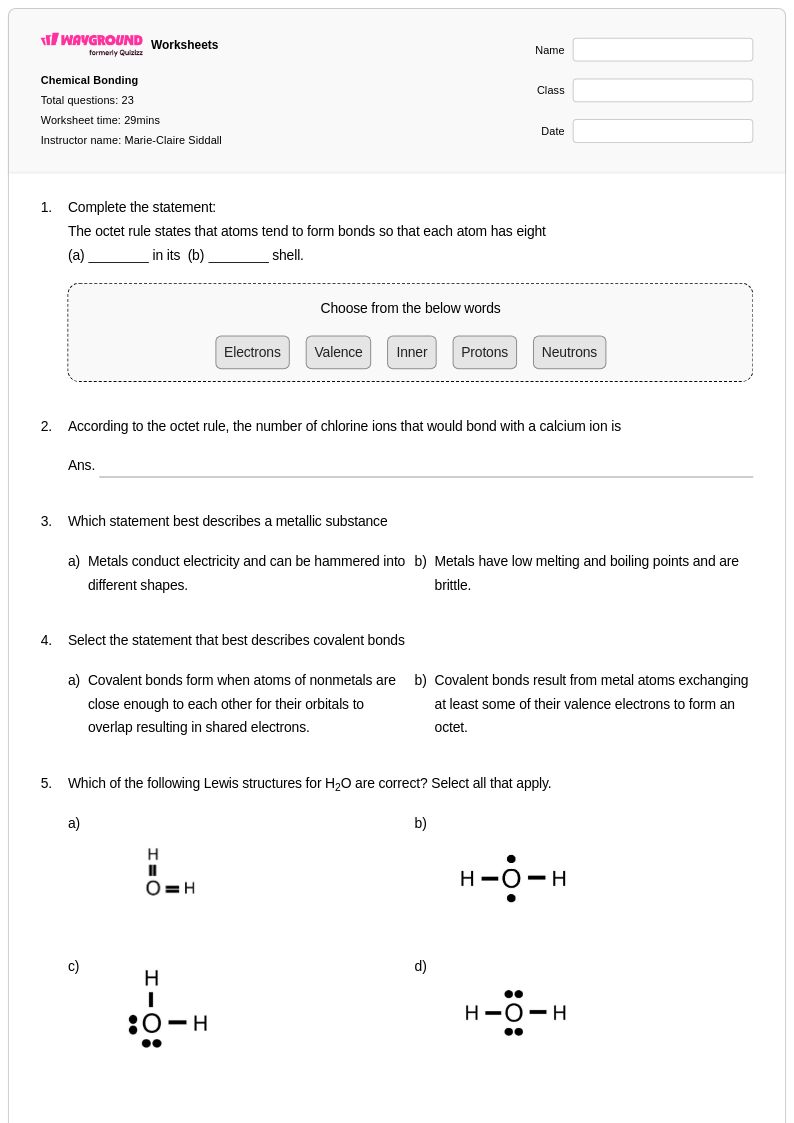
23 Q
10th - Uni

16 Q
9th - 12th
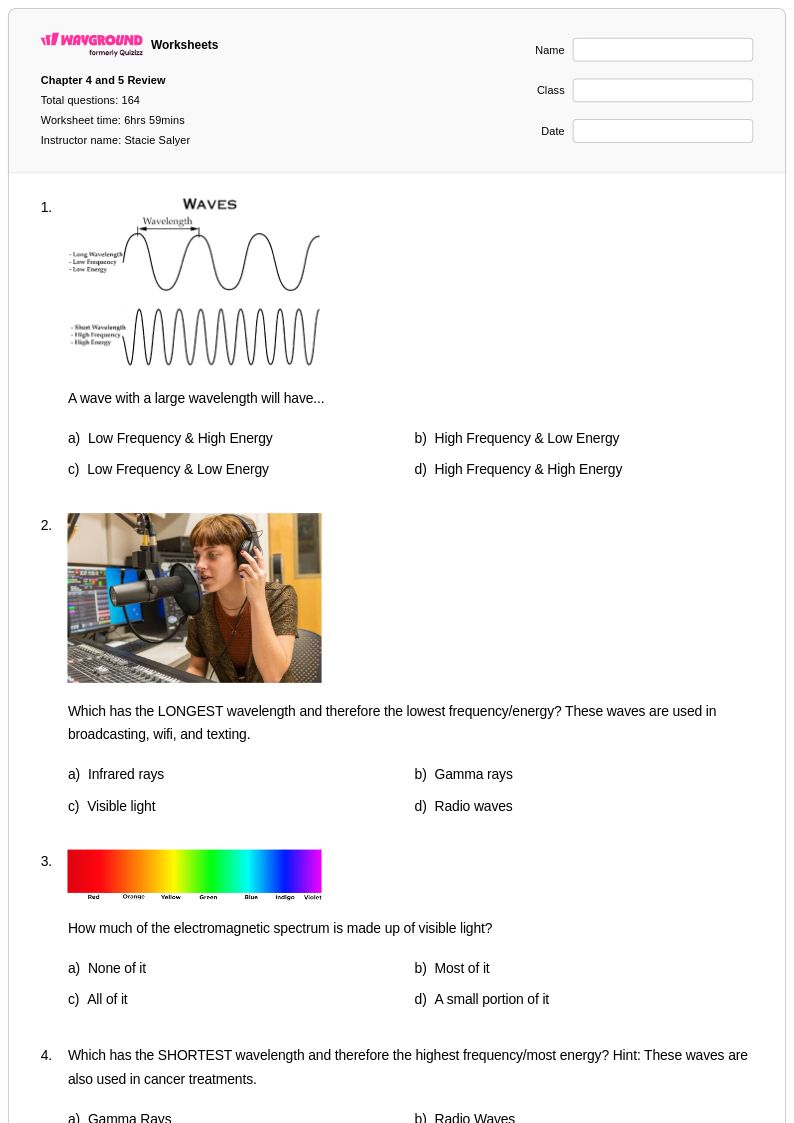
164 Q
12th

15 Q
9th - 12th

55 Q
9th - 12th
27 Q
9th - 12th

7 Q
12th
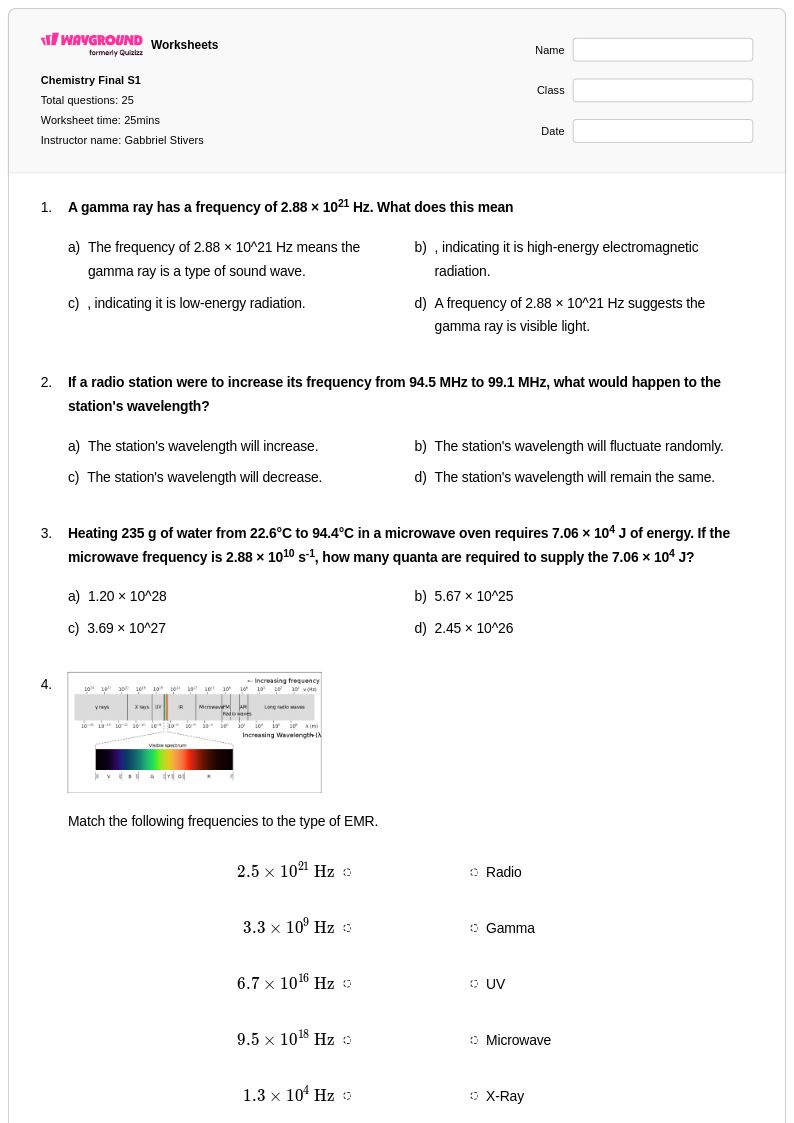
25 Q
9th - 12th
30 Q
9th - 12th
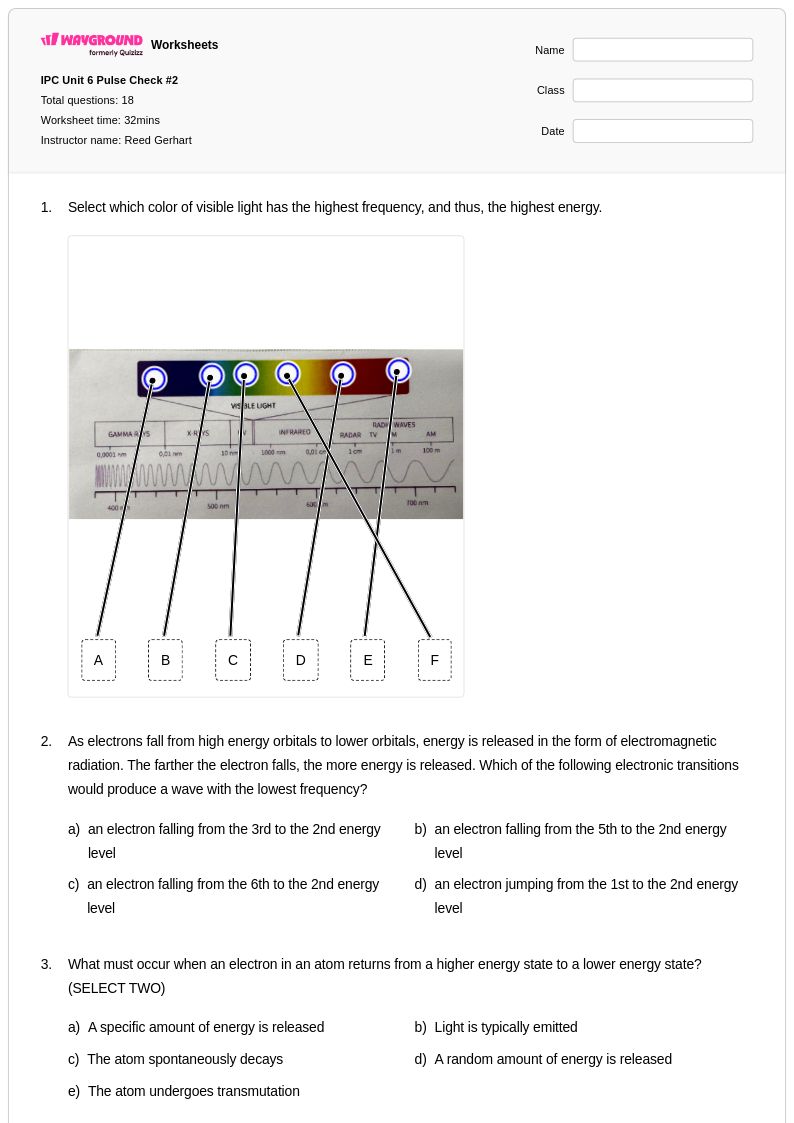
18 Q
9th - 12th

15 Q
9th - 12th
20 Q
9th - 12th

13 Q
9th - Uni

50 Q
9th - 12th

16 Q
5th - Uni
25 Q
10th - 12th

20 Q
9th - 12th

28 Q
9th - 12th

20 Q
9th - 12th

62 Q
9th - 12th
Explore Other Subject Worksheets for grade 12
Explore printable Orbitals worksheets for Grade 12
Grade 12 orbitals worksheets available through Wayground (formerly Quizizz) provide comprehensive coverage of atomic orbital theory, electron configuration, and molecular orbital concepts that are fundamental to advanced chemistry understanding. These expertly crafted resources strengthen students' abilities to visualize three-dimensional electron probability distributions, apply Hund's rule and the Aufbau principle, and predict molecular geometry using VSEPR theory. The practice problems systematically guide learners through complex topics including hybridization, sigma and pi bonding, and the relationship between orbital overlap and bond strength. Each worksheet includes detailed answer keys that support independent learning, while the free printable format ensures accessibility for all students as they master these challenging quantum mechanical concepts.
Wayground (formerly Quizizz) empowers chemistry teachers with millions of teacher-created orbital worksheets that can be easily searched, filtered, and customized to meet diverse classroom needs. The platform's robust collection includes resources aligned with advanced chemistry standards, offering both printable pdf versions for traditional instruction and digital formats for interactive learning experiences. Teachers can differentiate instruction by selecting worksheets that range from basic s, p, d, and f orbital identification to advanced molecular orbital diagrams and crystal field theory applications. These versatile tools support comprehensive lesson planning while providing targeted resources for remediation of struggling students and enrichment opportunities for advanced learners, ensuring that all Grade 12 students develop the spatial reasoning and theoretical understanding necessary for success in collegiate chemistry and related STEM fields.
