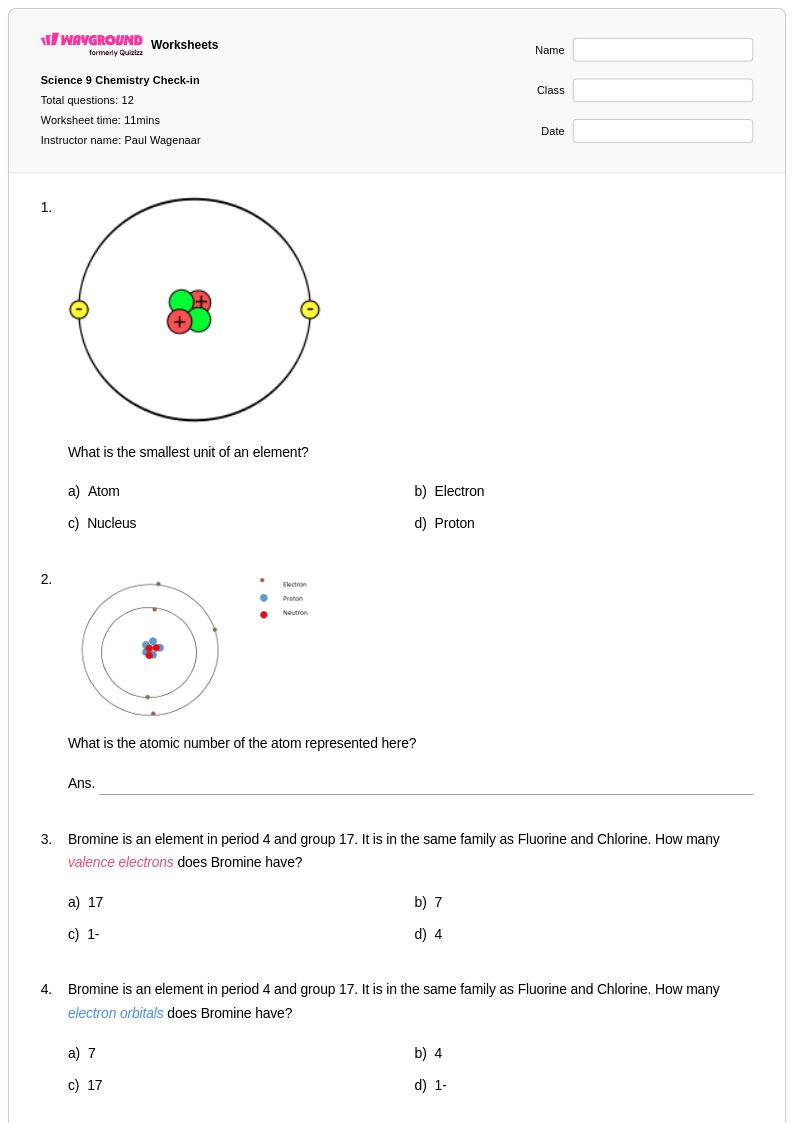
12 Q
9th

40 Q
9th

25 Q
9th

55 Q
9th - 12th

10 Q
9th
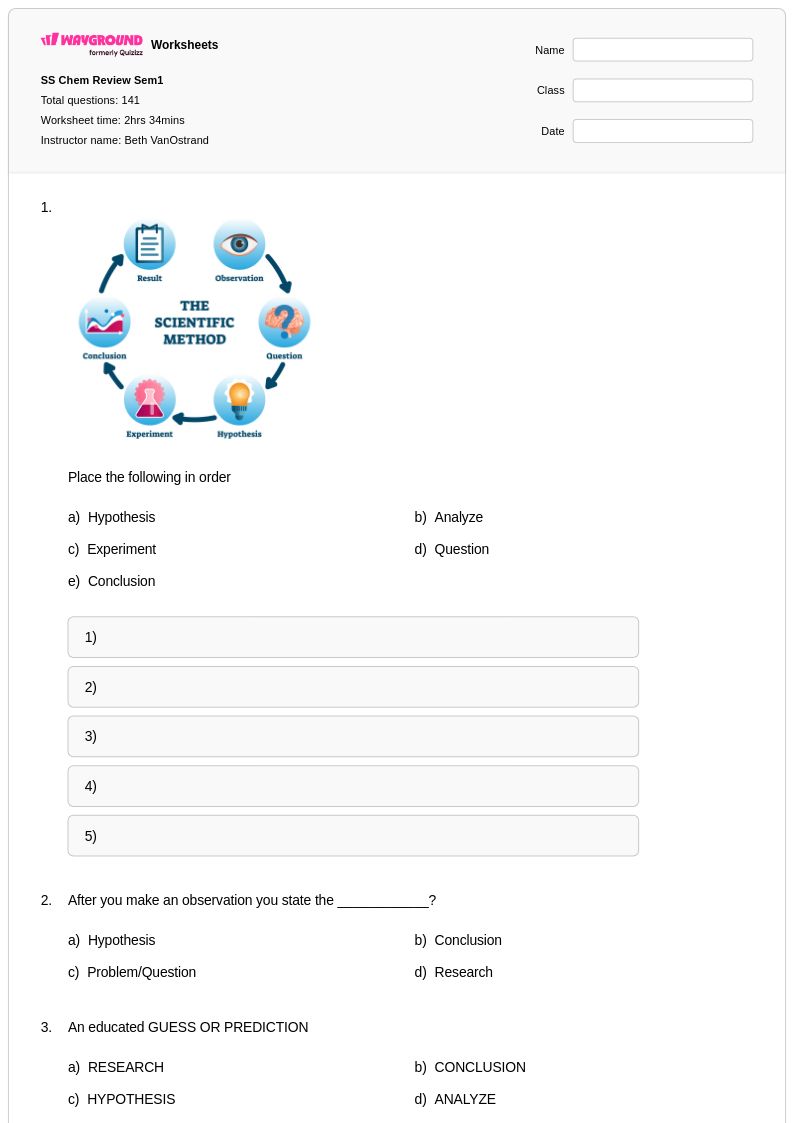
141 Q
9th - 12th
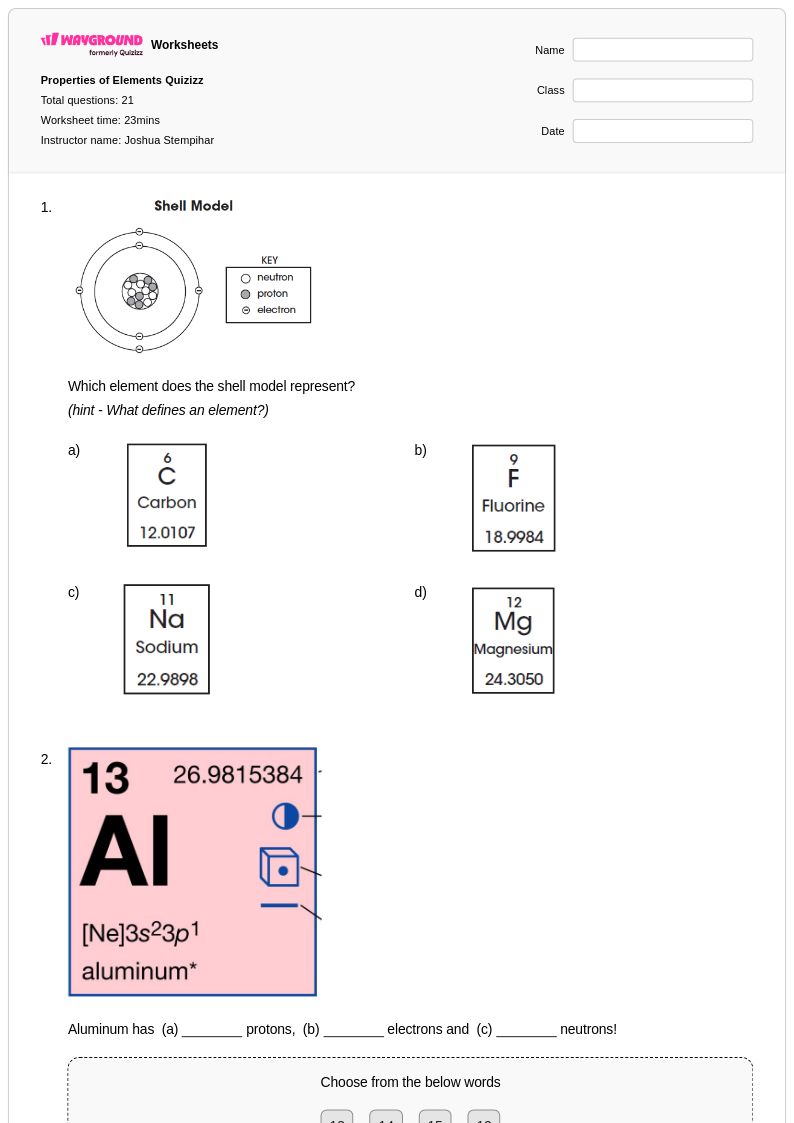
21 Q
9th

13 Q
9th - Uni
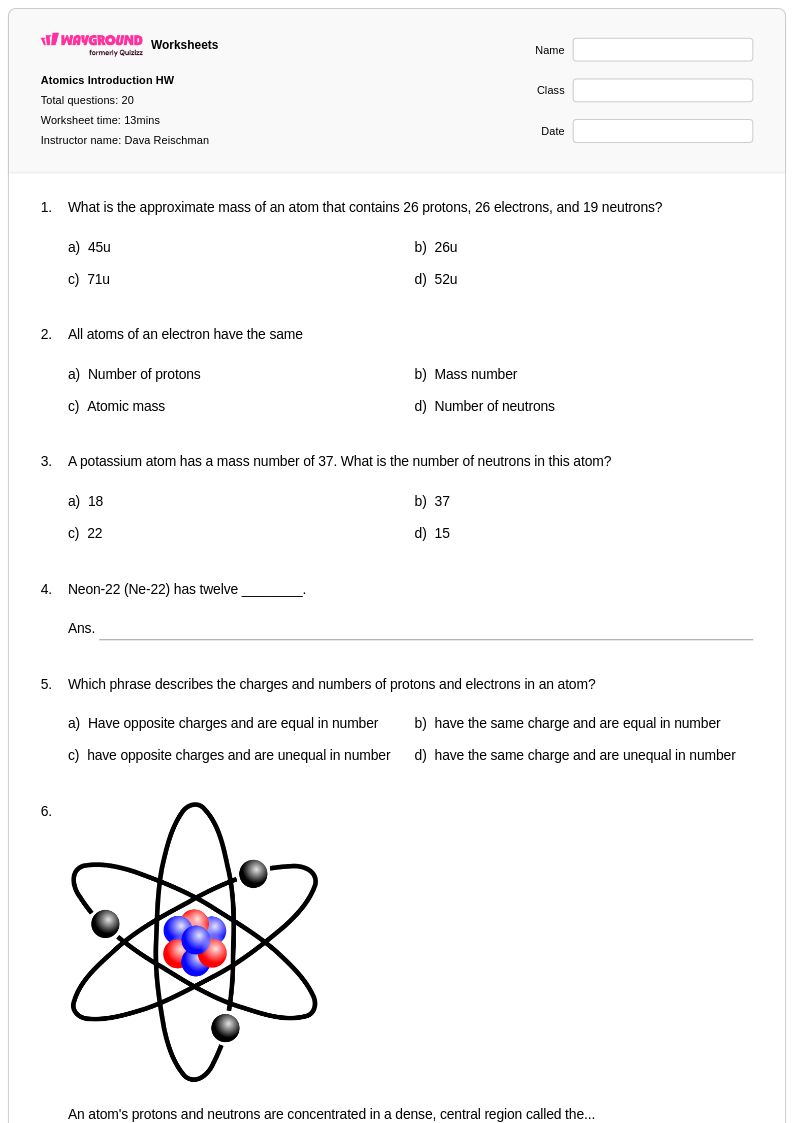
20 Q
9th - 12th
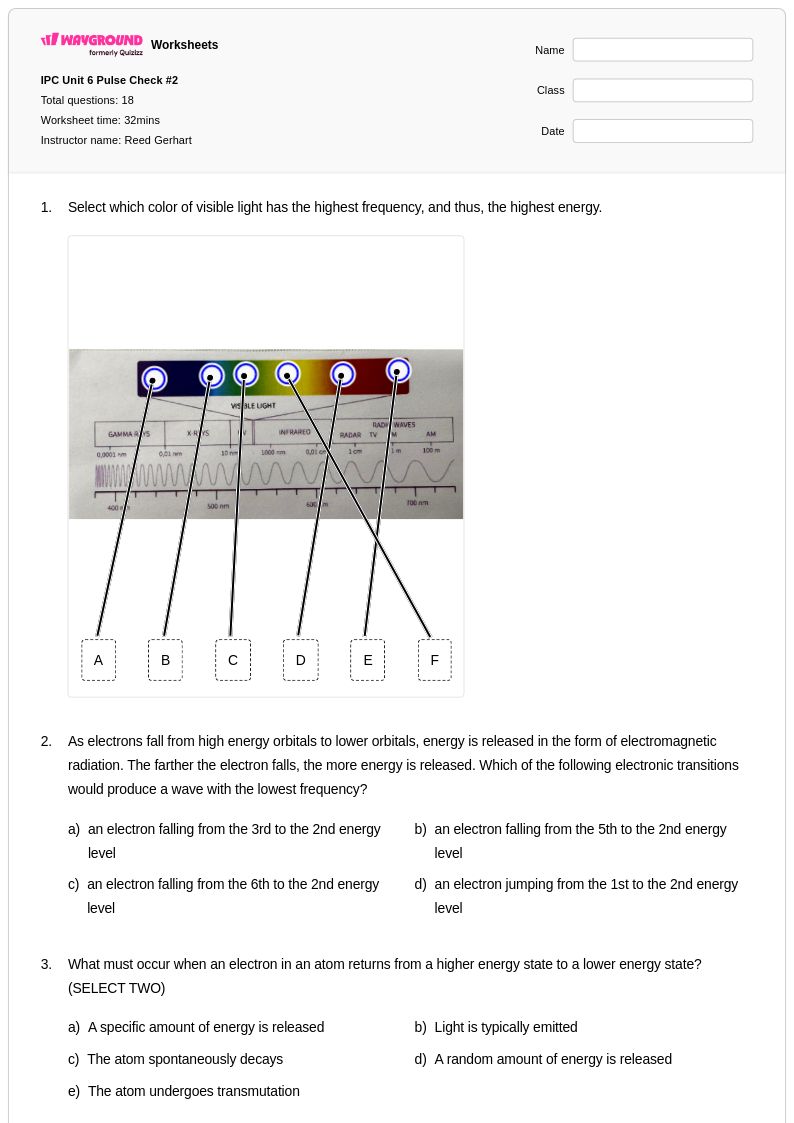
18 Q
9th - 12th

61 Q
9th

12 Q
9th

24 Q
9th

22 Q
9th - 12th
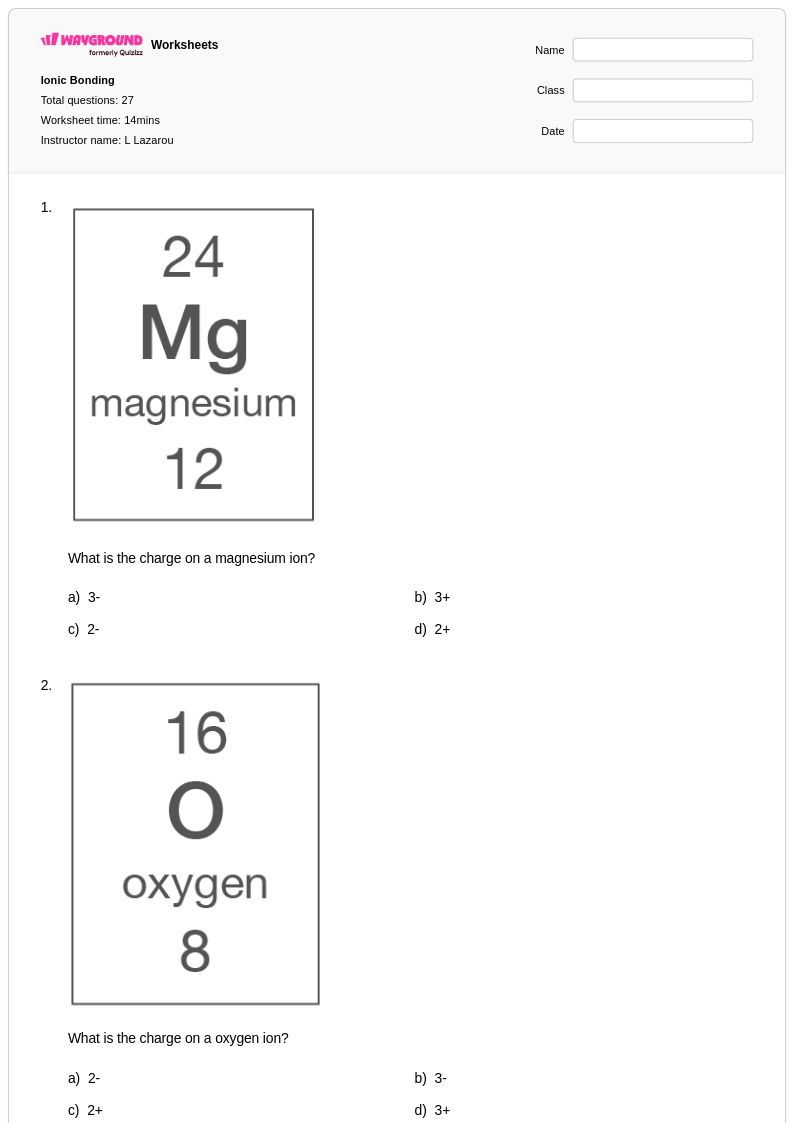
27 Q
9th
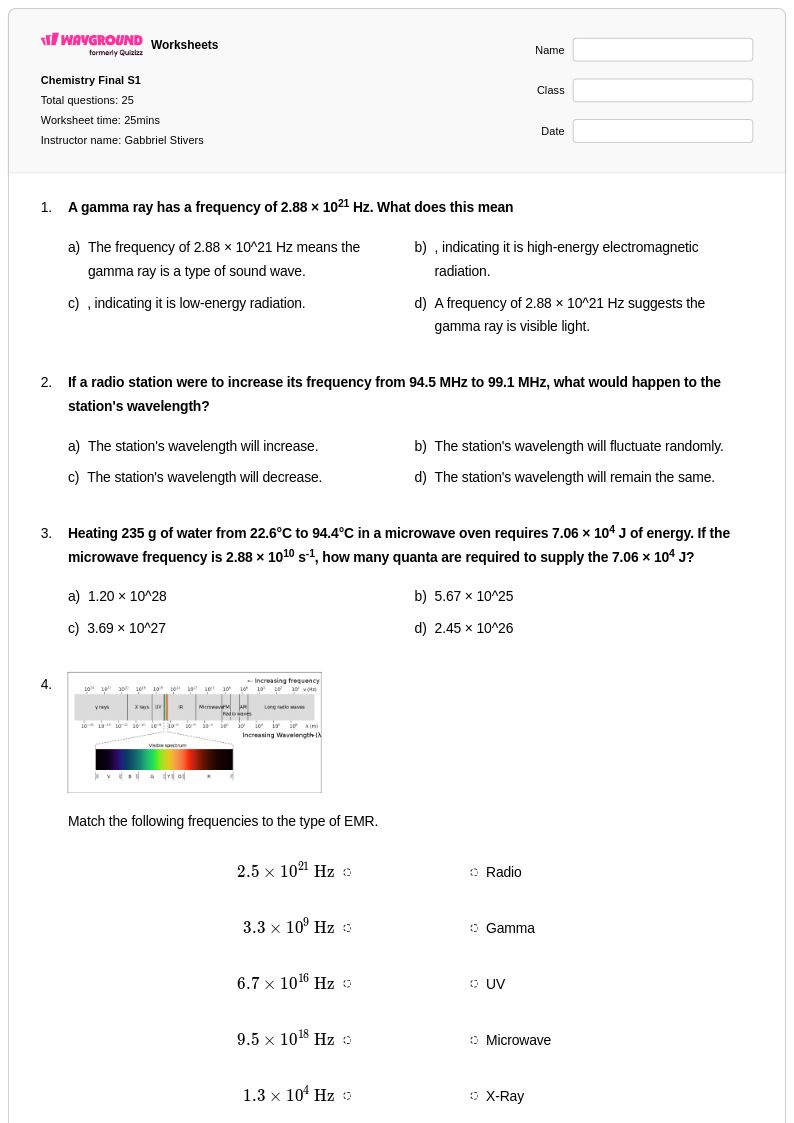
25 Q
9th - 12th

20 Q
9th
![2.1 Atomic structure and the Periodic Table [Must do] - Printable Electron-orbitals Worksheets Year 9 - Quizizz](https://quizizz-static.s3.amazonaws.com/_media/worksheets-new/64dc3b39e450d200098cb7cd-21-atomic-structure-and-the-periodic-table-must-do)
22 Q
9th
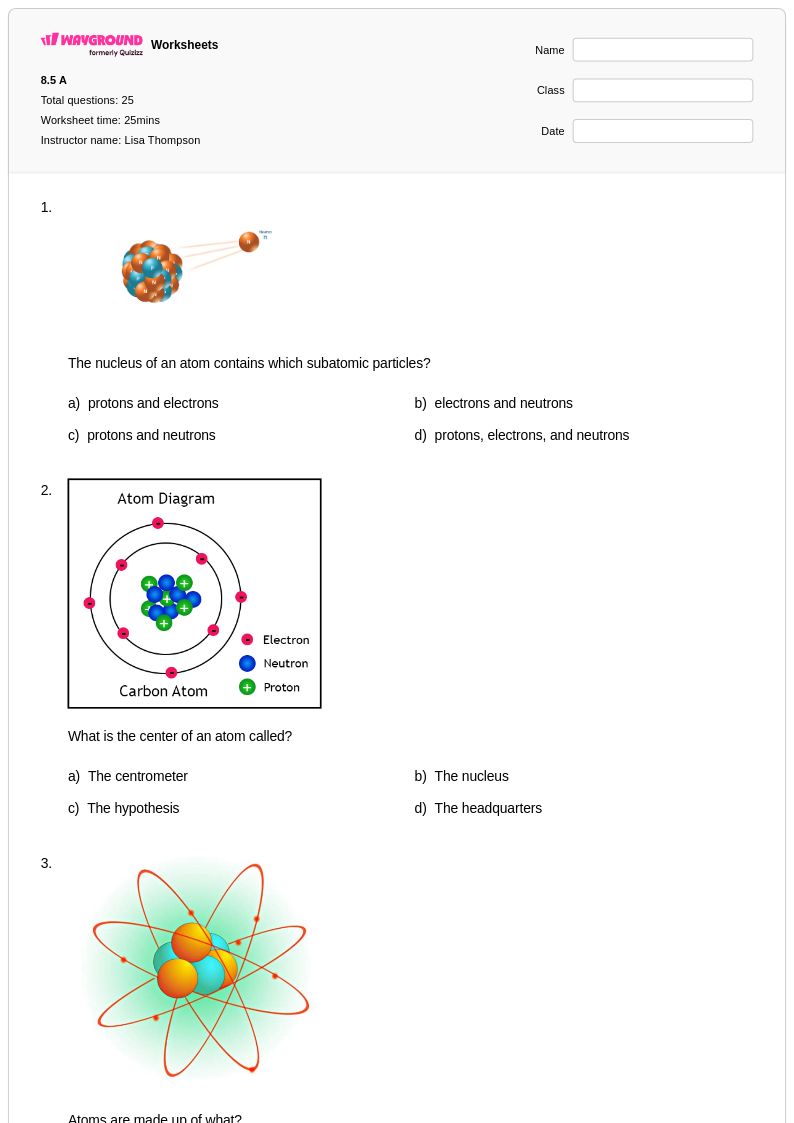
25 Q
8th - Uni

85 Q
9th

11 Q
8th - 9th

13 Q
9th
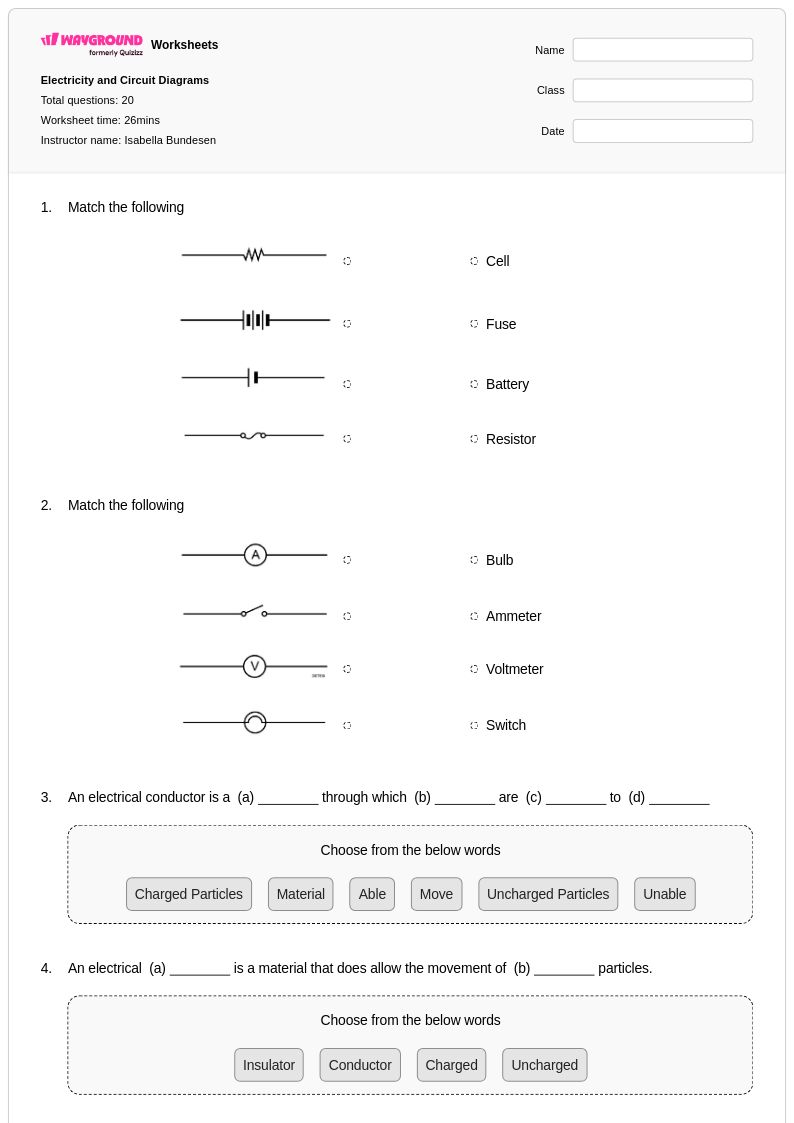
20 Q
9th
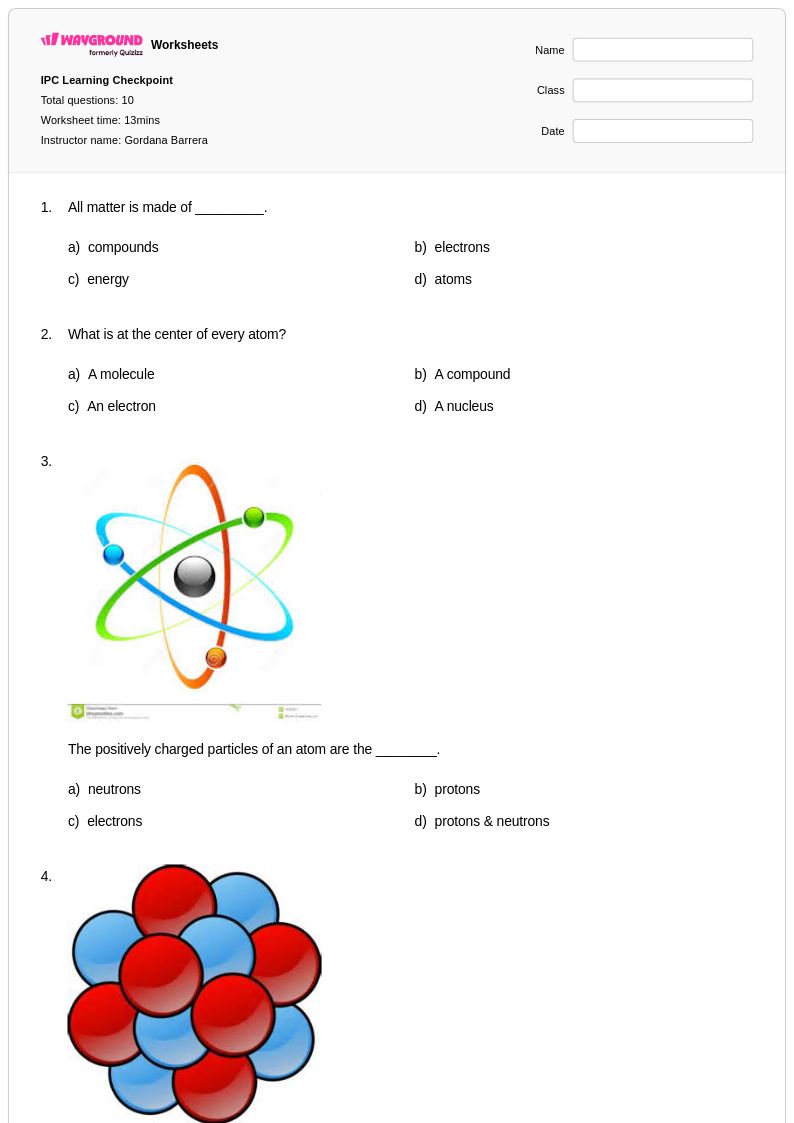
10 Q
9th - 12th
Explore Other Subject Worksheets for year 9
Explore printable Electron Orbitals worksheets for Year 9
Year 9 electron orbitals worksheets available through Wayground provide comprehensive practice materials that help students master one of chemistry's most fundamental yet challenging concepts. These carefully designed resources guide students through understanding atomic structure, orbital shapes, electron configurations, and energy levels while building critical thinking skills essential for advanced chemistry coursework. The collection includes varied practice problems that progress from basic orbital identification to complex electron arrangement scenarios, with each worksheet featuring detailed answer keys that support independent learning and self-assessment. Teachers can access these free printables in convenient PDF format, making it simple to distribute materials for classroom instruction, homework assignments, or exam preparation while ensuring students develop a solid foundation in atomic theory.
Wayground's extensive library of teacher-created electron orbitals resources offers millions of high-quality materials that streamline lesson planning and support differentiated instruction for Year 9 chemistry educators. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific learning standards and customize content to match diverse student needs and ability levels. These versatile materials are available in both printable and digital formats, enabling seamless integration into traditional classroom settings or online learning environments while providing flexibility for remediation, enrichment, and targeted skill practice. The comprehensive collection empowers educators to effectively scaffold complex atomic concepts, ensuring all students can progress from basic orbital recognition to sophisticated understanding of electron behavior and chemical bonding principles.
