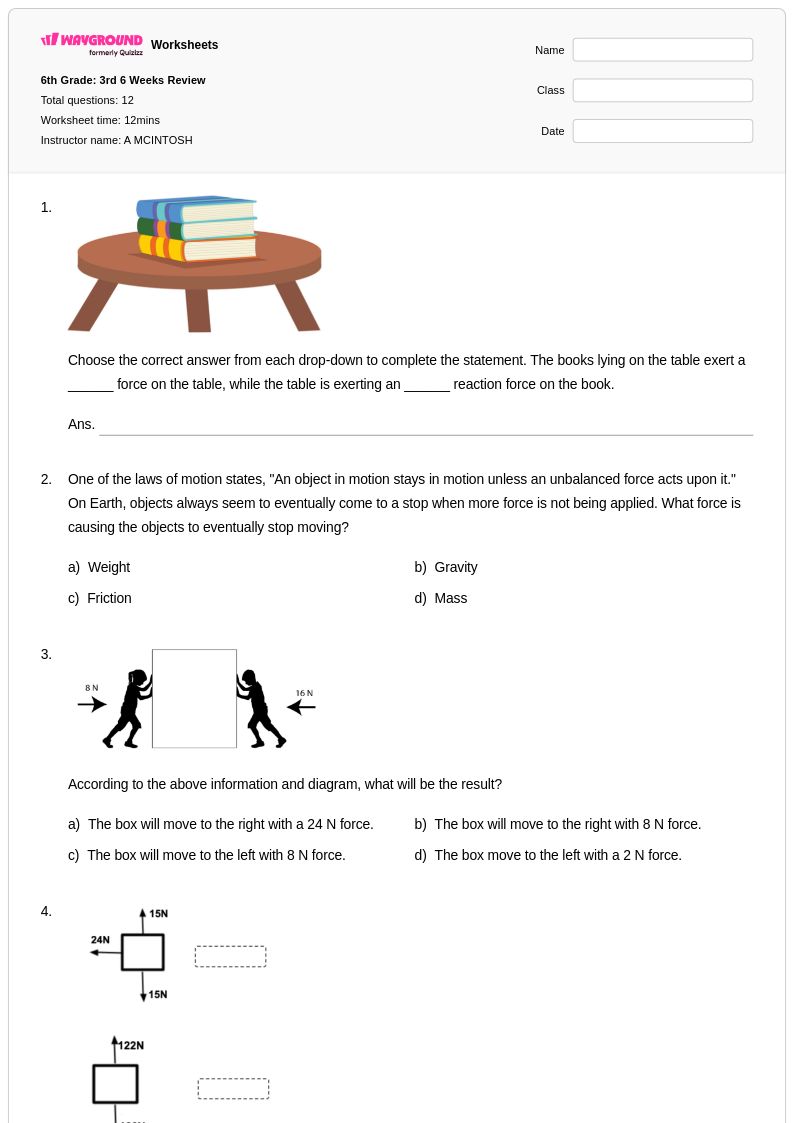
12Q
6th

51Q
6th - 8th

23Q
6th

14Q
6th - 8th
35Q
6th
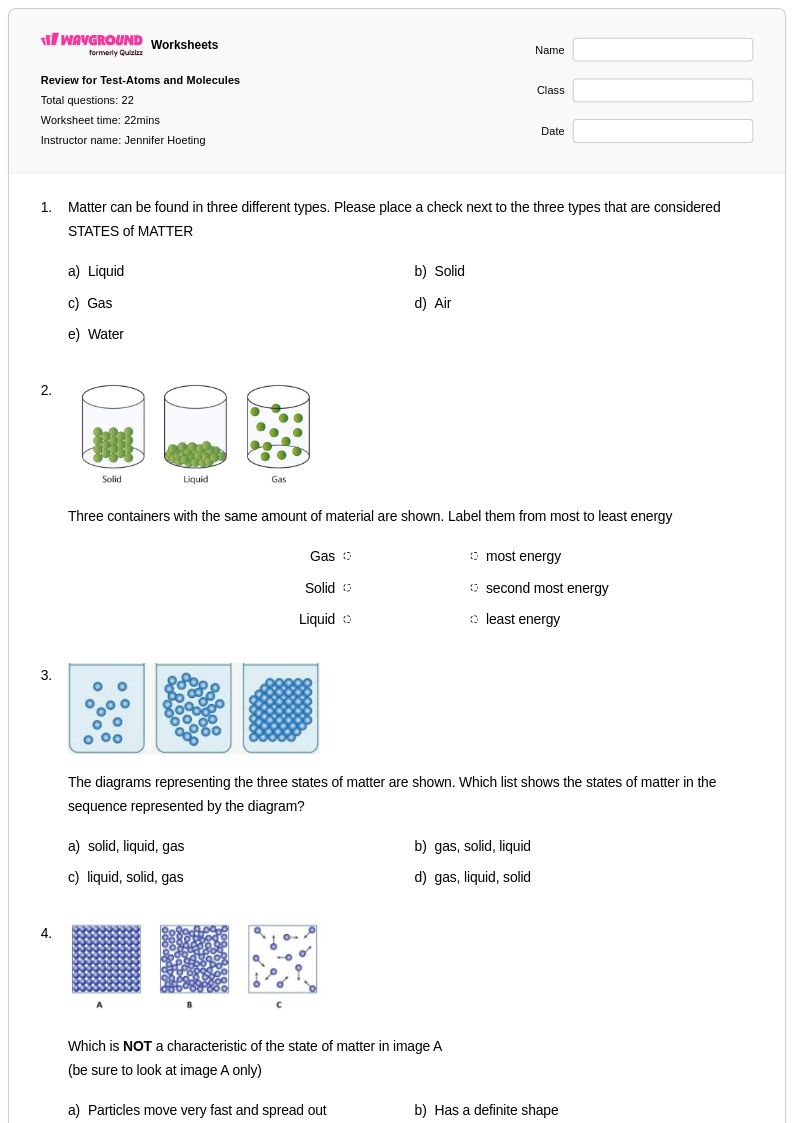
22Q
6th

20Q
6th - 8th
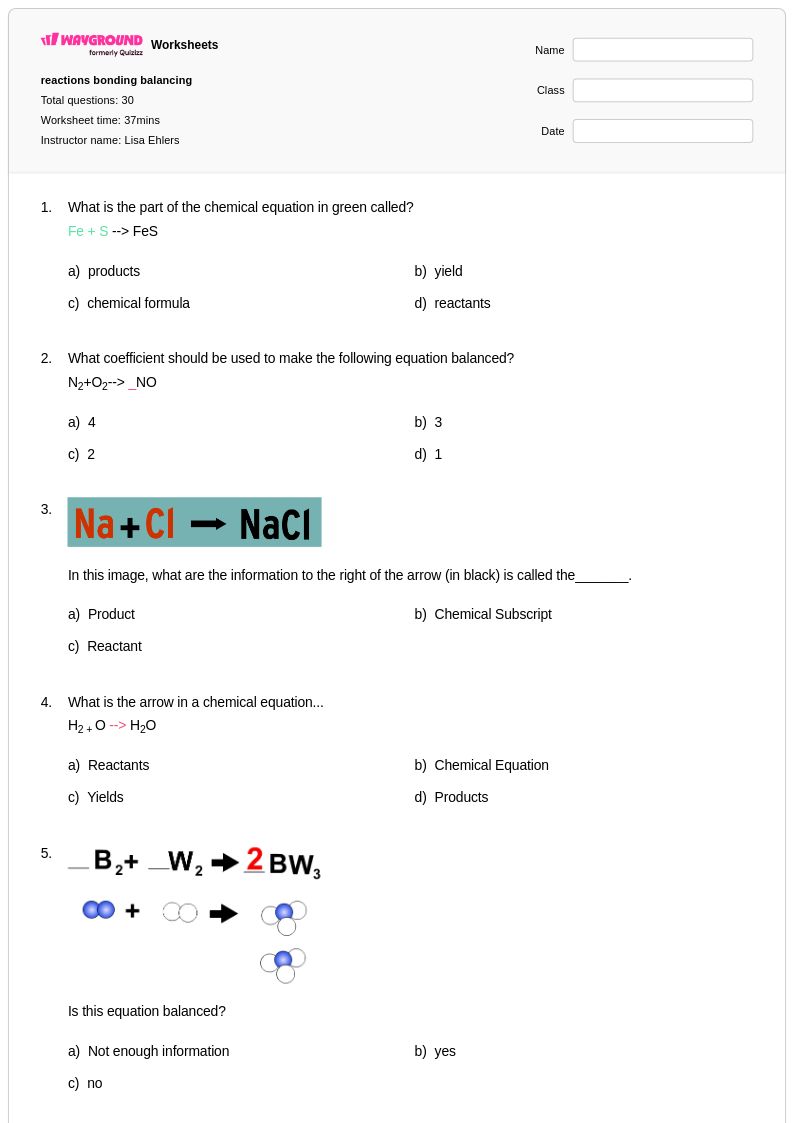
30Q
6th - 8th
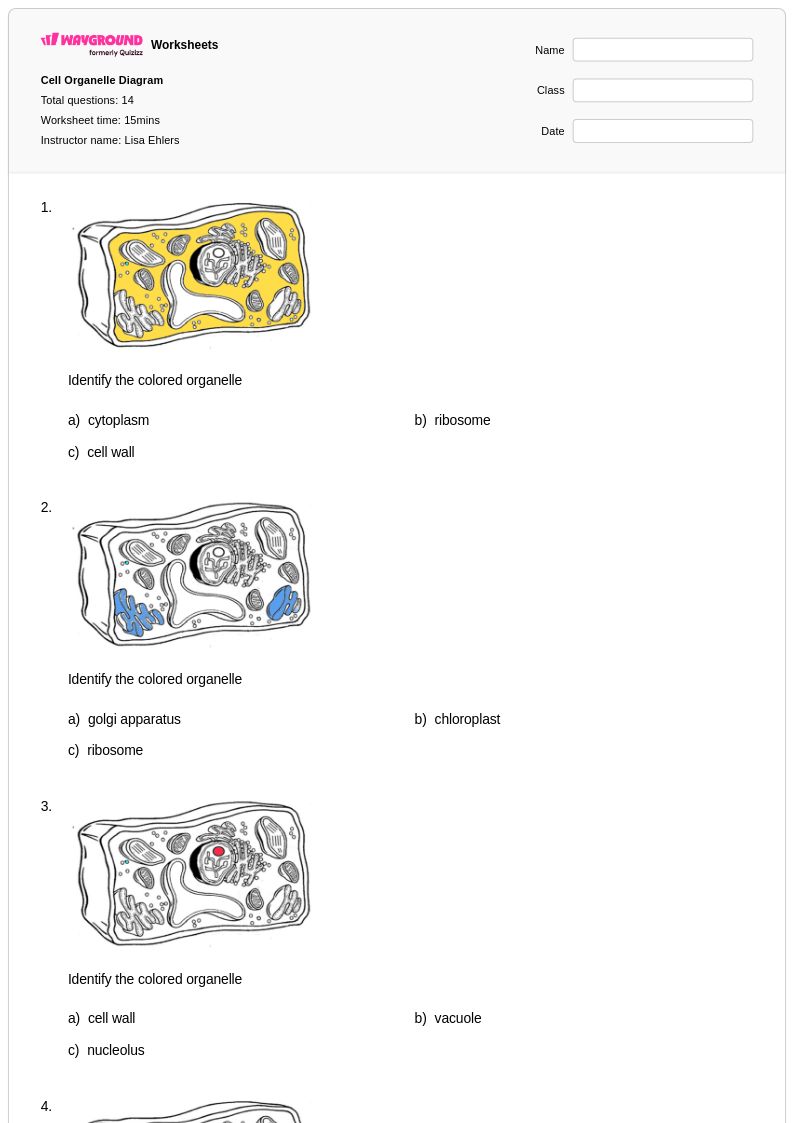
14Q
6th - 8th
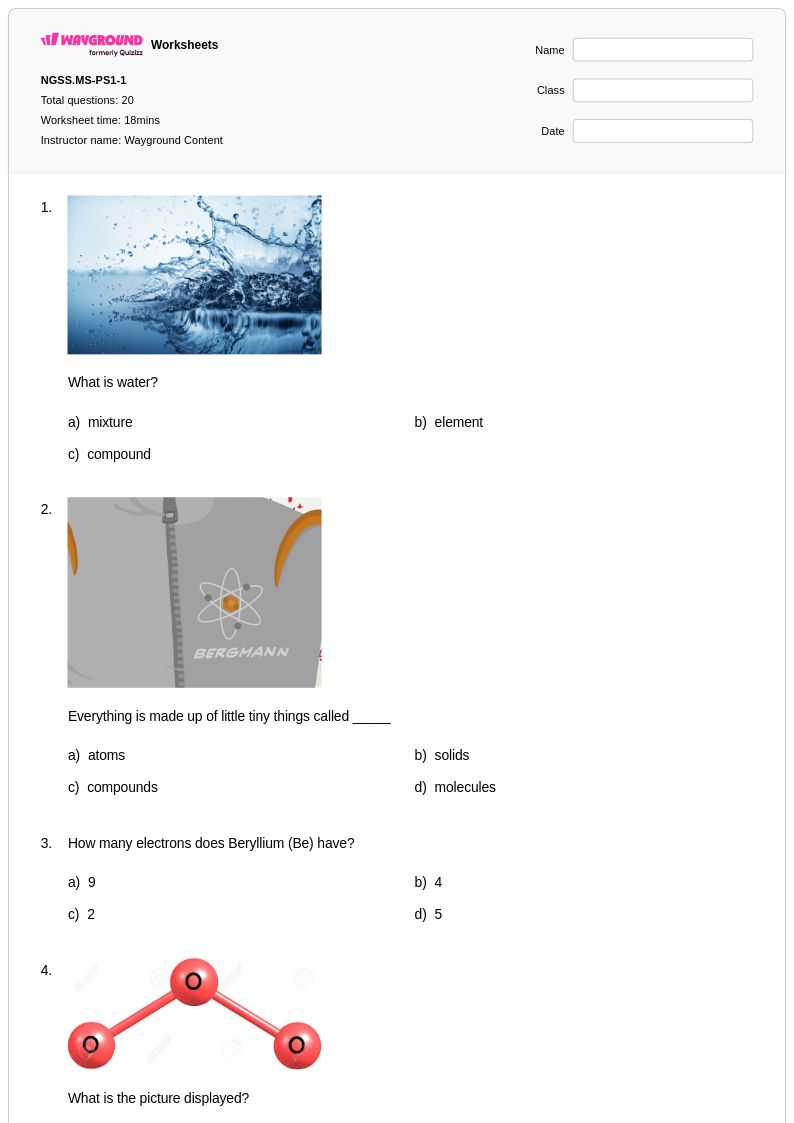
20Q
6th - 8th
22Q
6th
15Q
6th

15Q
6th

32Q
6th - 8th

15Q
6th - 8th
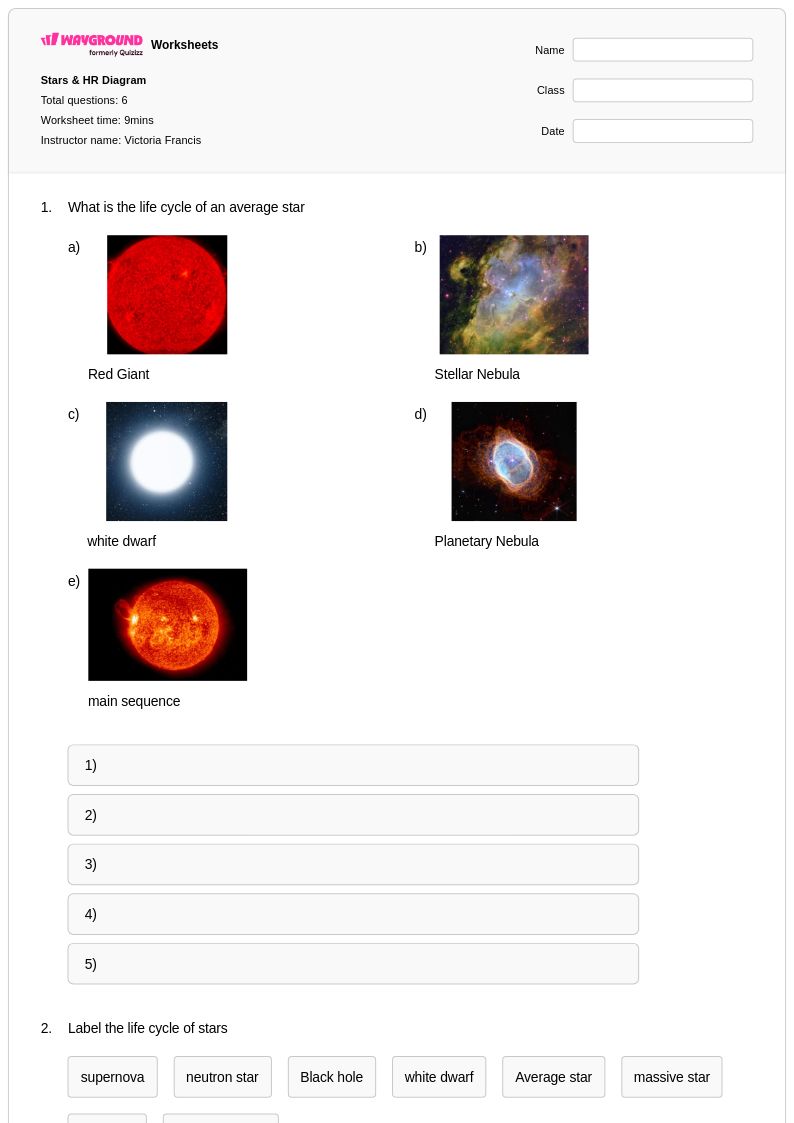
6Q
6th - 8th
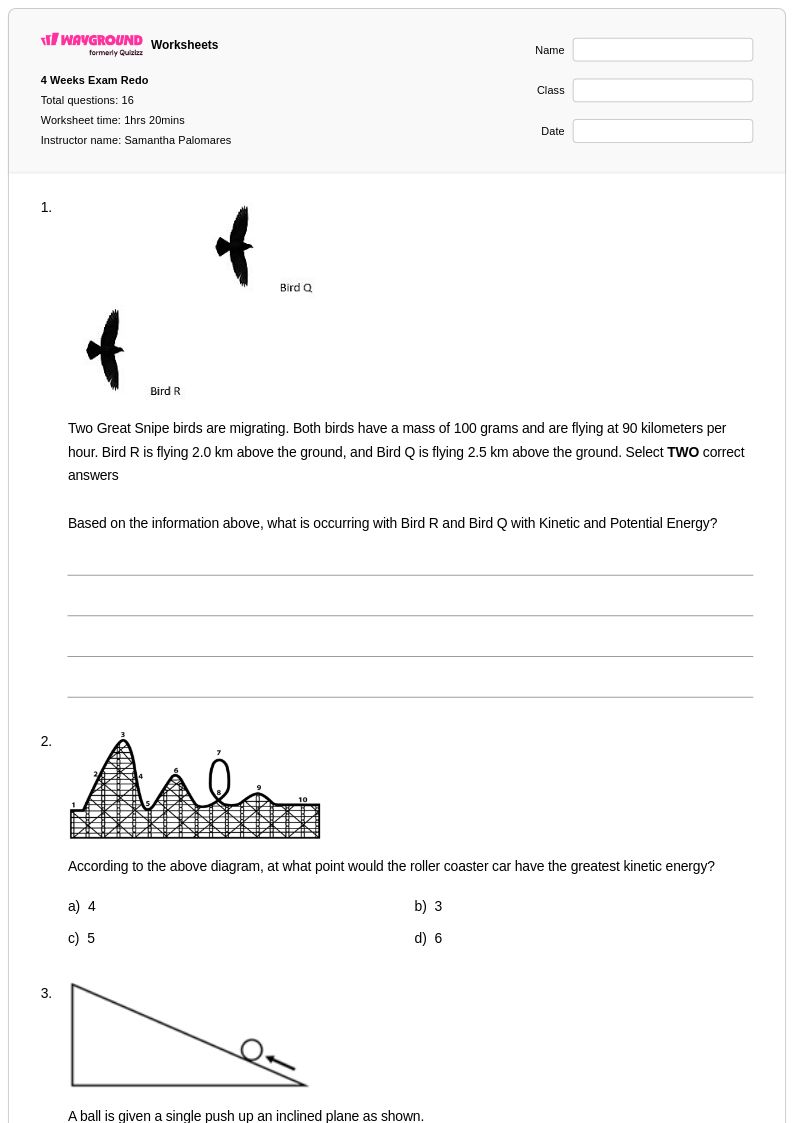
16Q
6th
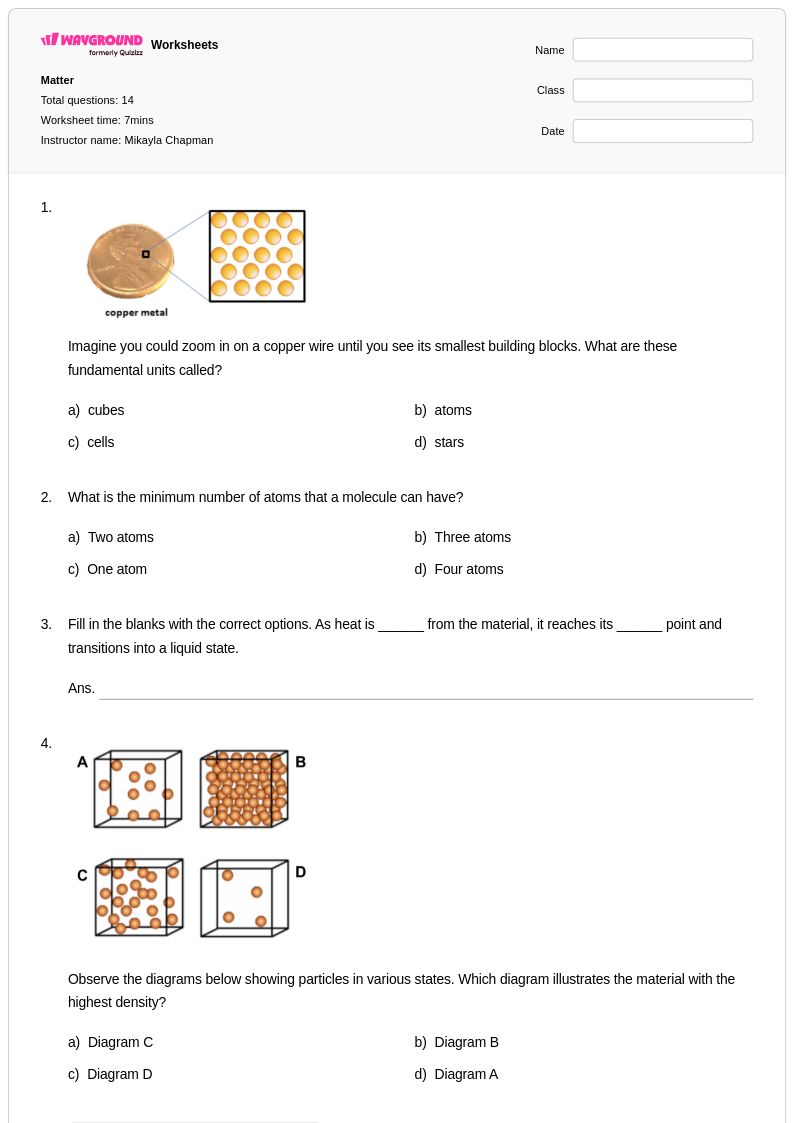
14Q
6th
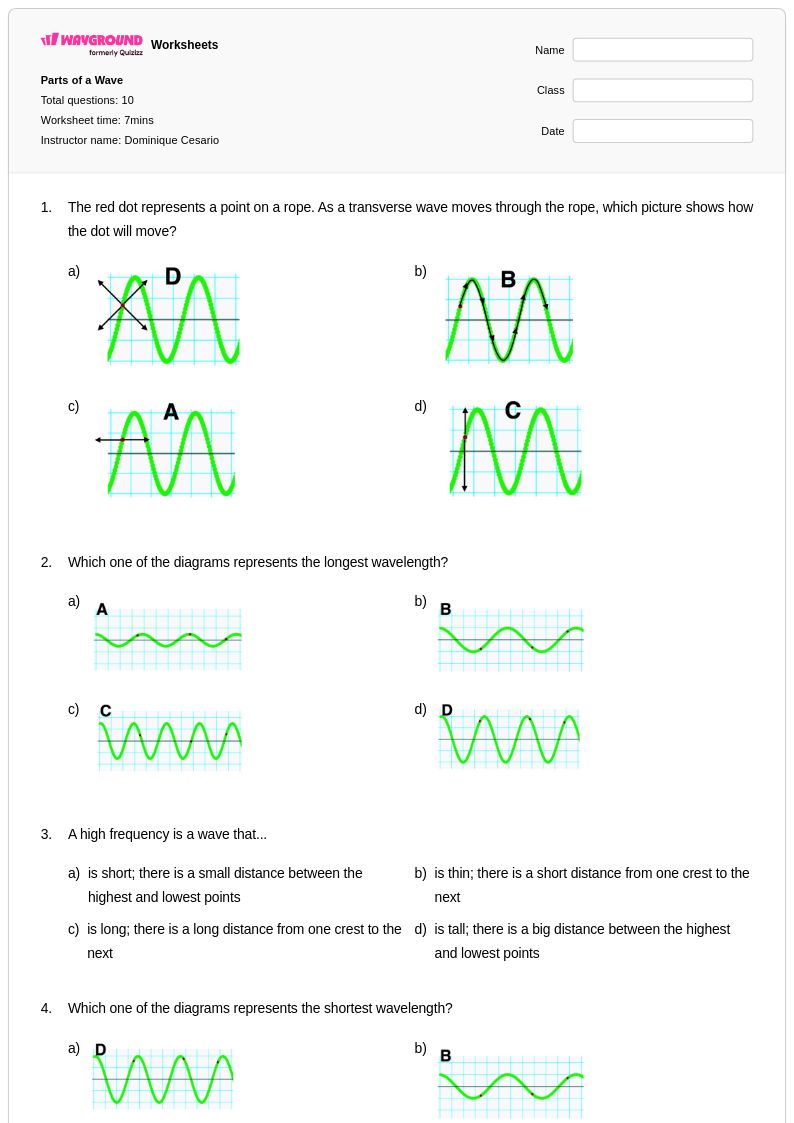
10Q
6th

20Q
4th - 6th

18Q
6th

24Q
6th
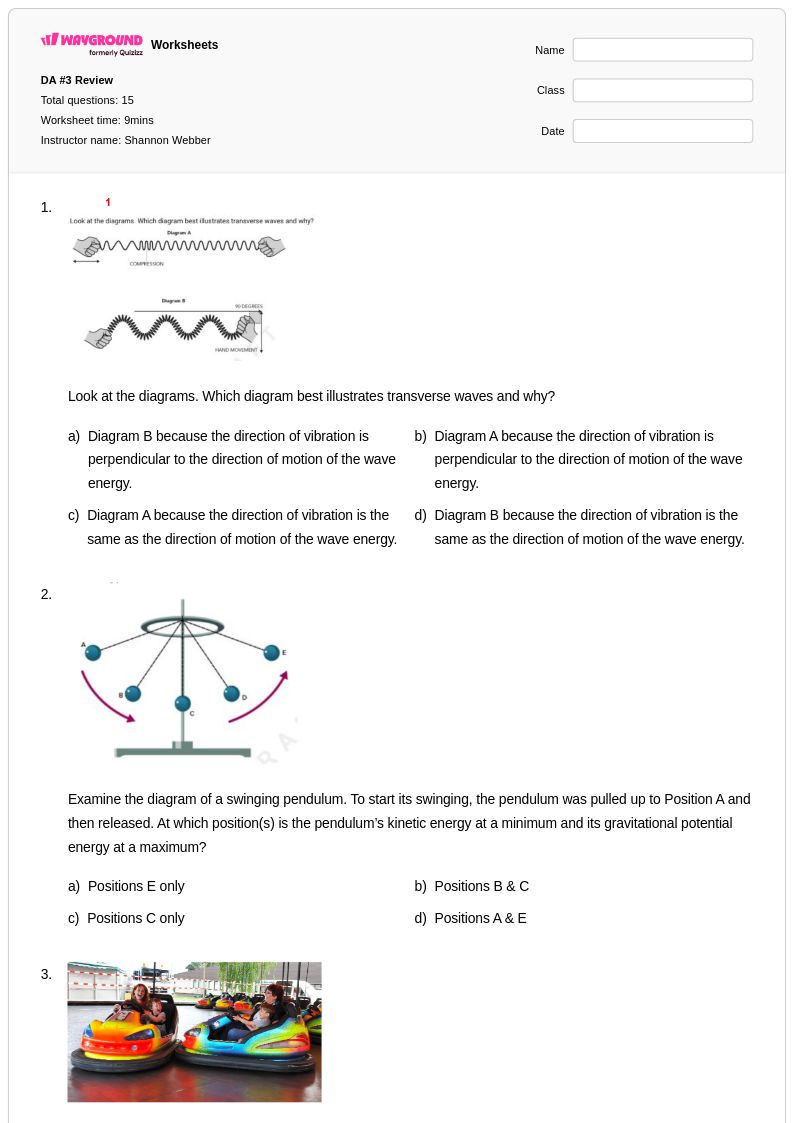
15Q
6th
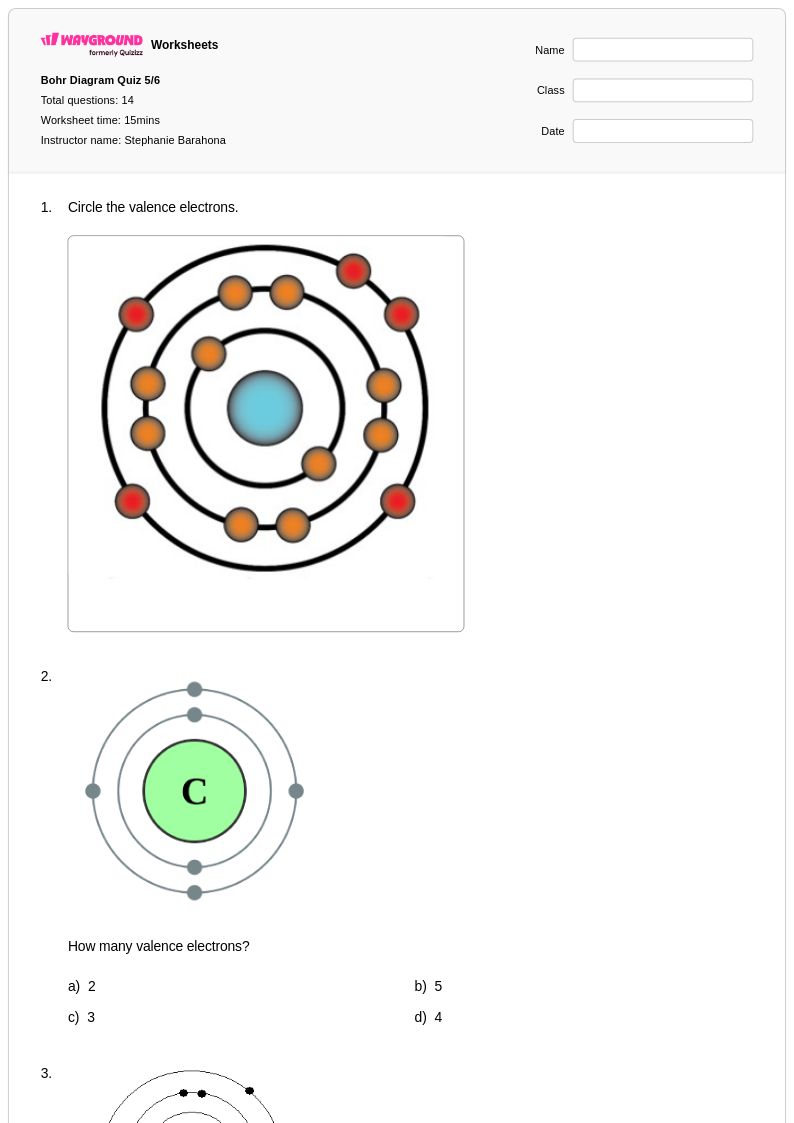
14Q
6th - 8th
Explorar Lewis Dot Diagram hojas de trabajo por grados
Explore otras hojas de trabajo de materias para class 6
Explore printable Lewis Dot Diagram worksheets for Class 6
Lewis dot diagrams provide Class 6 students with a foundational understanding of atomic structure and chemical bonding through visual representation of valence electrons. Wayground's comprehensive collection of Lewis dot diagram worksheets helps students master the essential skill of drawing electron configurations for atoms and simple molecules, building critical thinking abilities needed for advanced chemistry concepts. These carefully crafted practice problems guide students through step-by-step processes for determining valence electrons, placing dots around element symbols, and understanding how atoms achieve stability. The free printable worksheets include detailed answer keys that support independent learning and self-assessment, while pdf formats ensure consistent formatting across different devices and printing options.
Wayground (formerly Quizizz) empowers educators with millions of teacher-created Lewis dot diagram resources specifically designed for Class 6 science instruction. The platform's advanced search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific learning standards and differentiate instruction based on individual student needs. These customizable digital and printable materials support diverse classroom applications, from initial concept introduction and guided practice to remediation for struggling learners and enrichment activities for advanced students. Teachers can seamlessly modify existing worksheets or combine multiple resources to create comprehensive lesson plans that address varying skill levels, ensuring every student develops confidence in drawing Lewis structures and understanding electron behavior in atoms and molecules.
