Recommended Topics for you
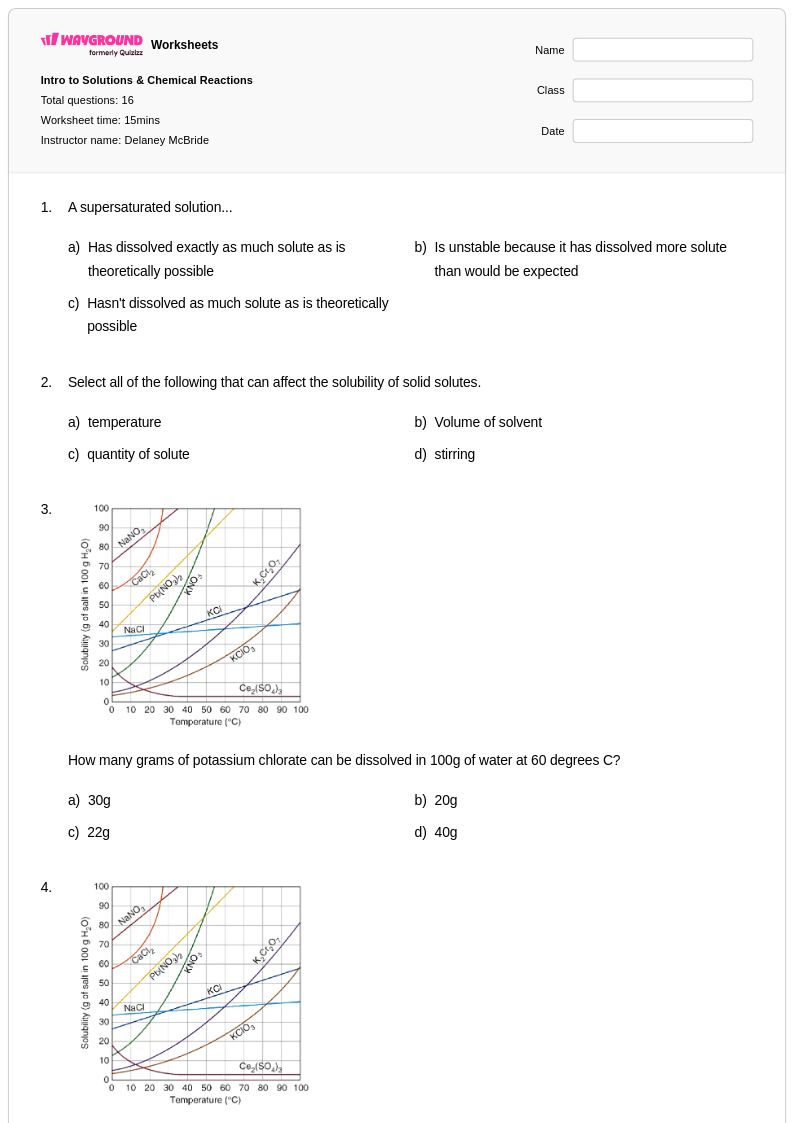
16 Q
9th - 12th
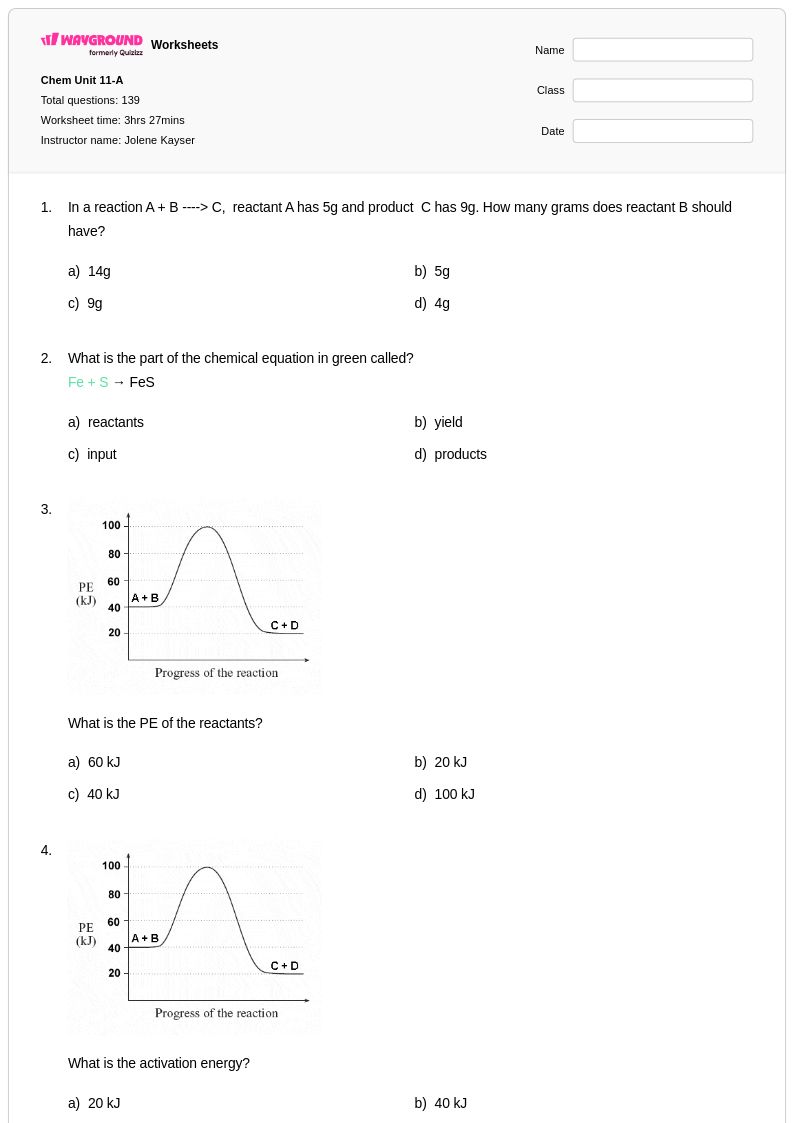
139 Q
9th - 12th

25 Q
8th - Uni
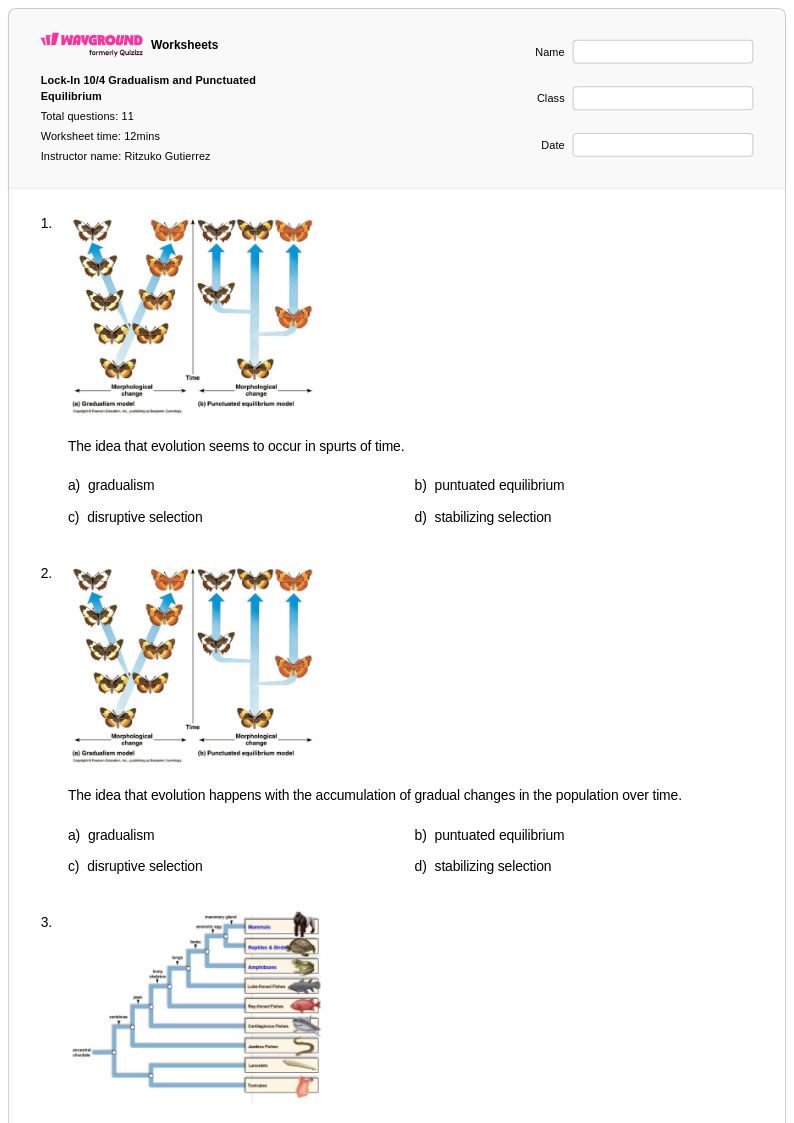
11 Q
12th
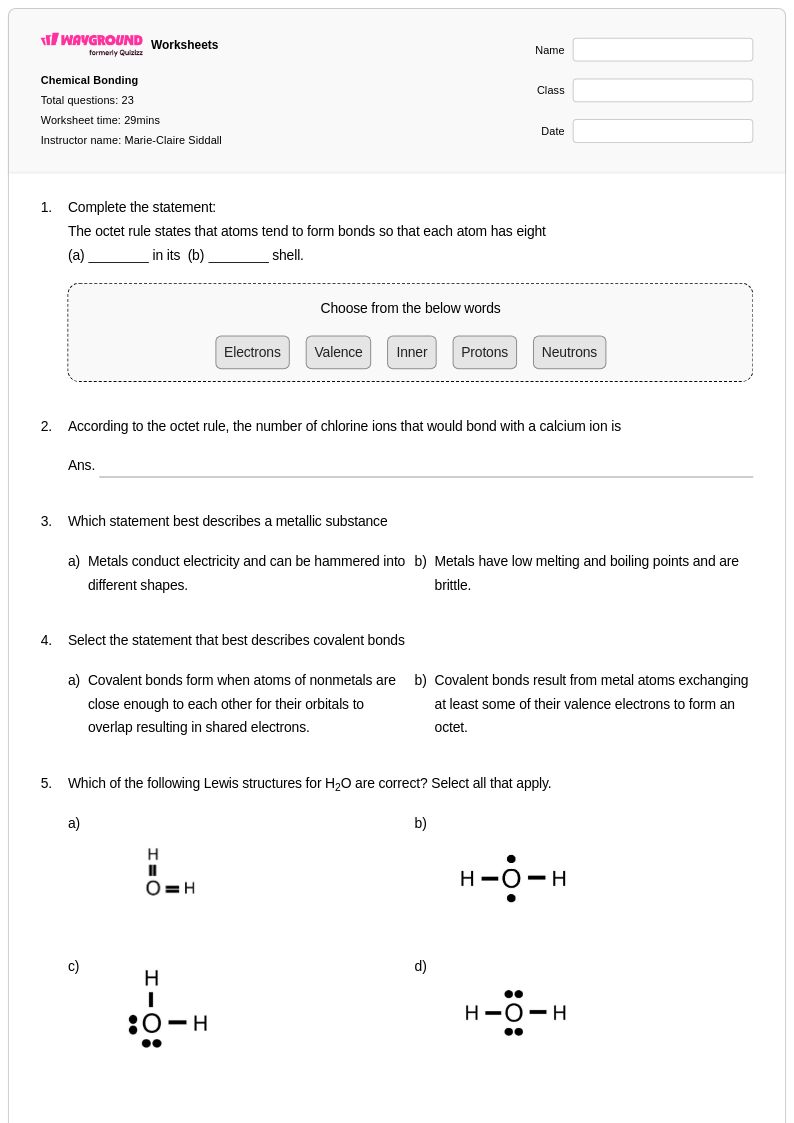
23 Q
10th - Uni

17 Q
7th - Uni
15 Q
8th - Uni
14 Q
6th - Uni

25 Q
9th - Uni
15 Q
8th - Uni

18 Q
12th

94 Q
12th
14 Q
9th - 12th

12 Q
12th
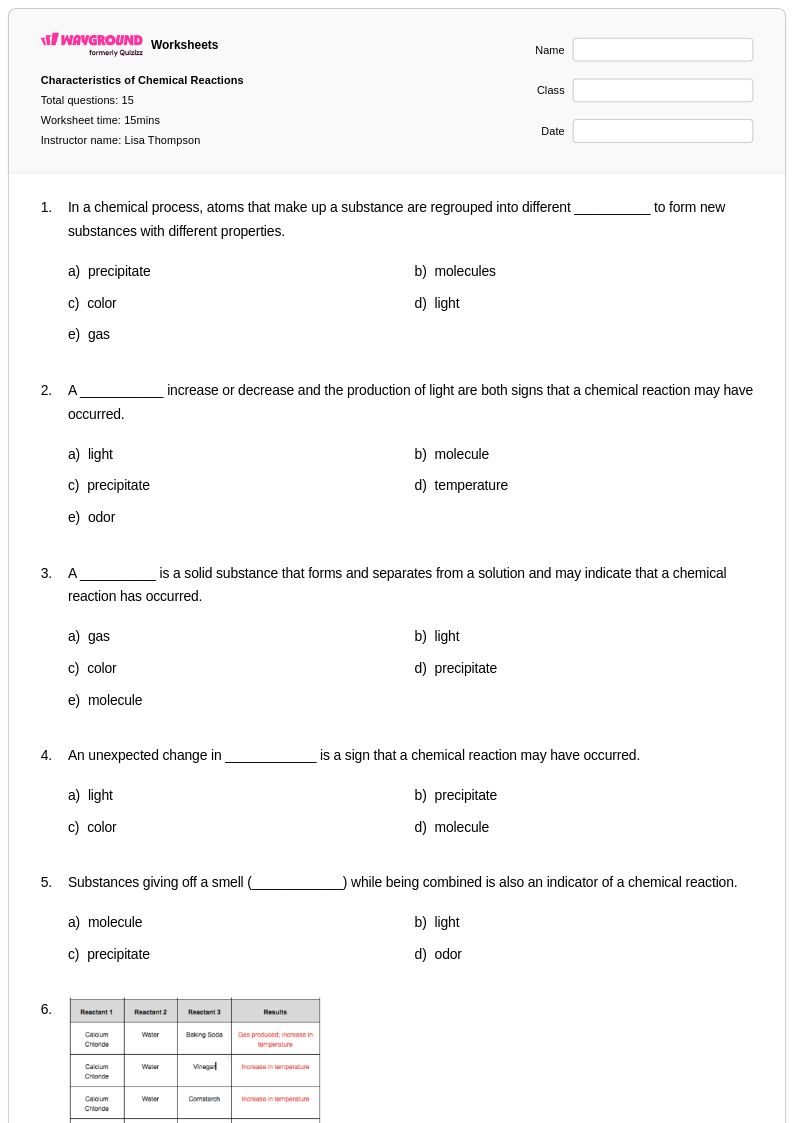
15 Q
7th - Uni

25 Q
8th - Uni

25 Q
6th - Uni

12 Q
12th

14 Q
9th - 12th
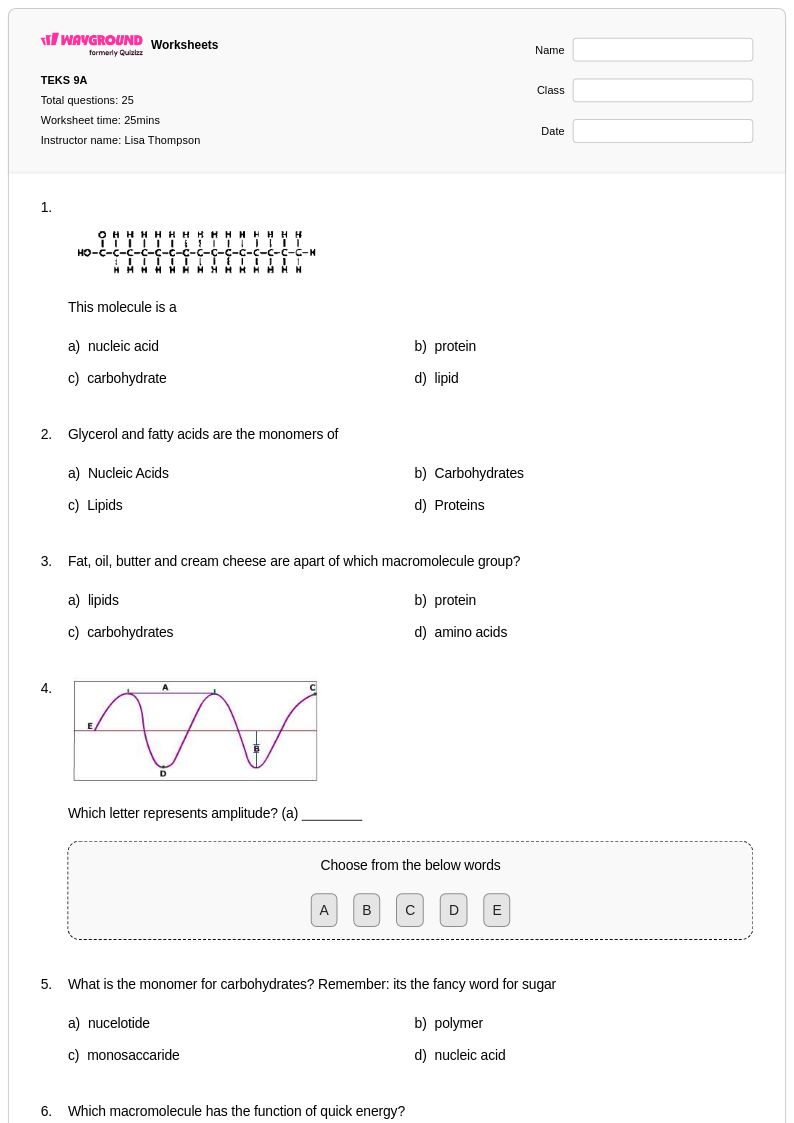
25 Q
9th - Uni
15 Q
5th - Uni

56 Q
12th
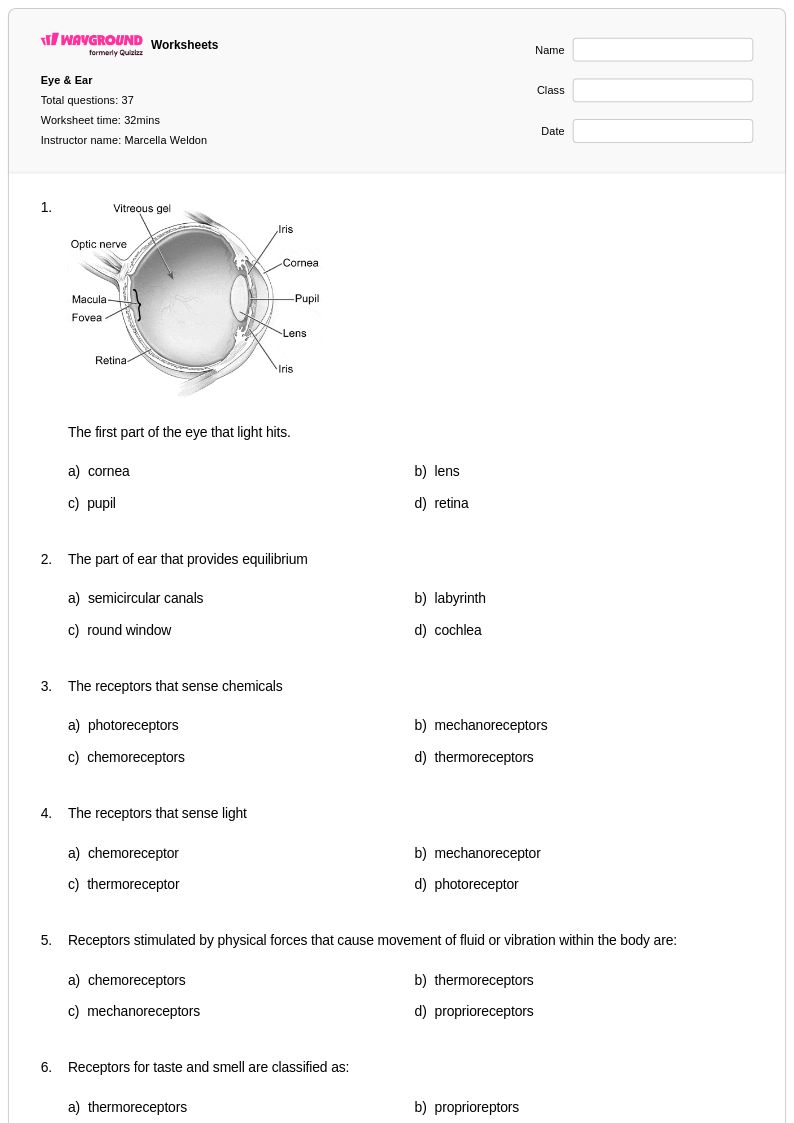
37 Q
12th

15 Q
6th - Uni
Explore Chemical Equilibrium Worksheets for year 12 by Topic
Explore Other Subject Worksheets for year 12
Explore printable Chemical Equilibrium worksheets for Year 12
Chemical equilibrium worksheets for Year 12 students available through Wayground (formerly Quizizz) provide comprehensive practice with the fundamental principles governing reversible reactions and dynamic balance in chemical systems. These expertly crafted resources strengthen students' understanding of equilibrium constants, Le Chatelier's principle, reaction quotients, and the factors that influence equilibrium positions including temperature, pressure, and concentration changes. Students work through practice problems that challenge them to calculate equilibrium expressions, predict shifts in equilibrium, and analyze ICE tables for complex reaction systems. Each worksheet includes detailed answer keys that guide students through step-by-step solutions, while the free printable format ensures accessibility for both classroom instruction and independent study.
Wayground (formerly Quizizz) supports chemistry educators with an extensive collection of millions of teacher-created resources specifically designed for Year 12 chemical equilibrium instruction. The platform's advanced search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific curriculum standards and learning objectives, while built-in differentiation tools enable customization based on individual student needs and skill levels. These chemical equilibrium materials are available in both printable pdf formats and interactive digital versions, providing flexibility for diverse classroom environments and teaching styles. Teachers utilize these resources for targeted skill practice, remediation of challenging concepts like equilibrium calculations, and enrichment activities that extend student understanding of industrial applications such as the Haber process and contact process for sulfuric acid production.
