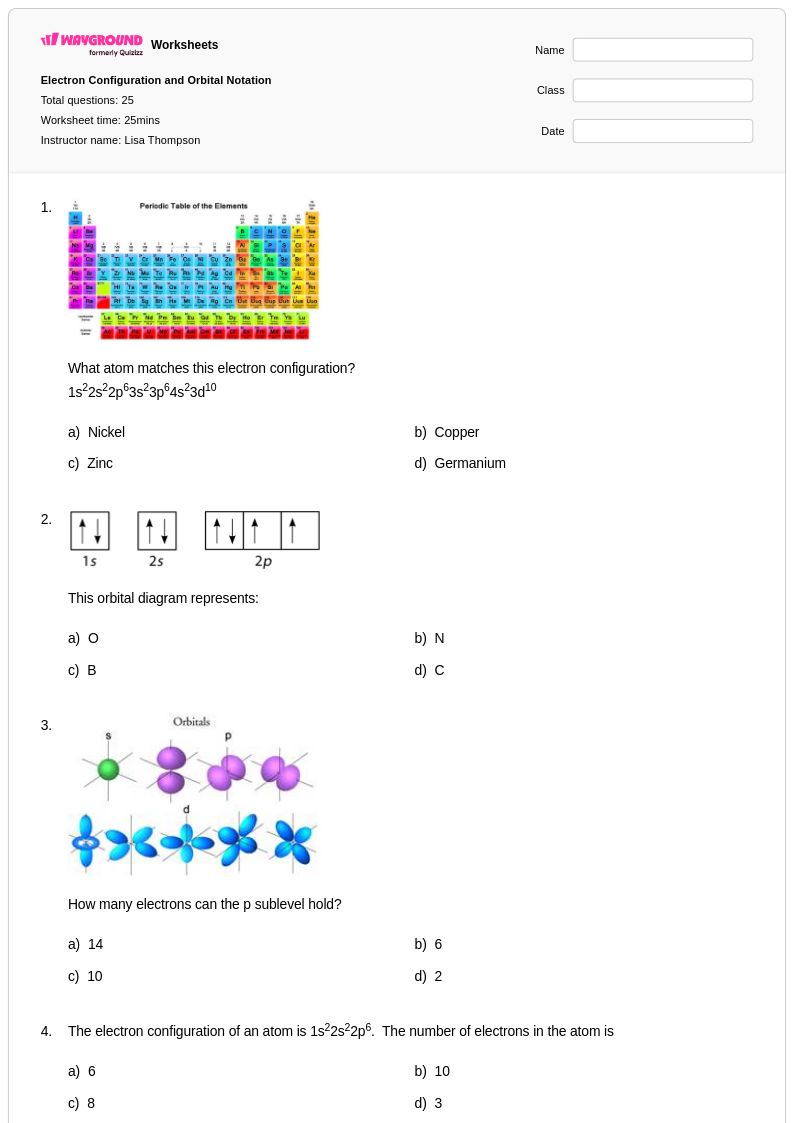
25 Q
11th - Uni
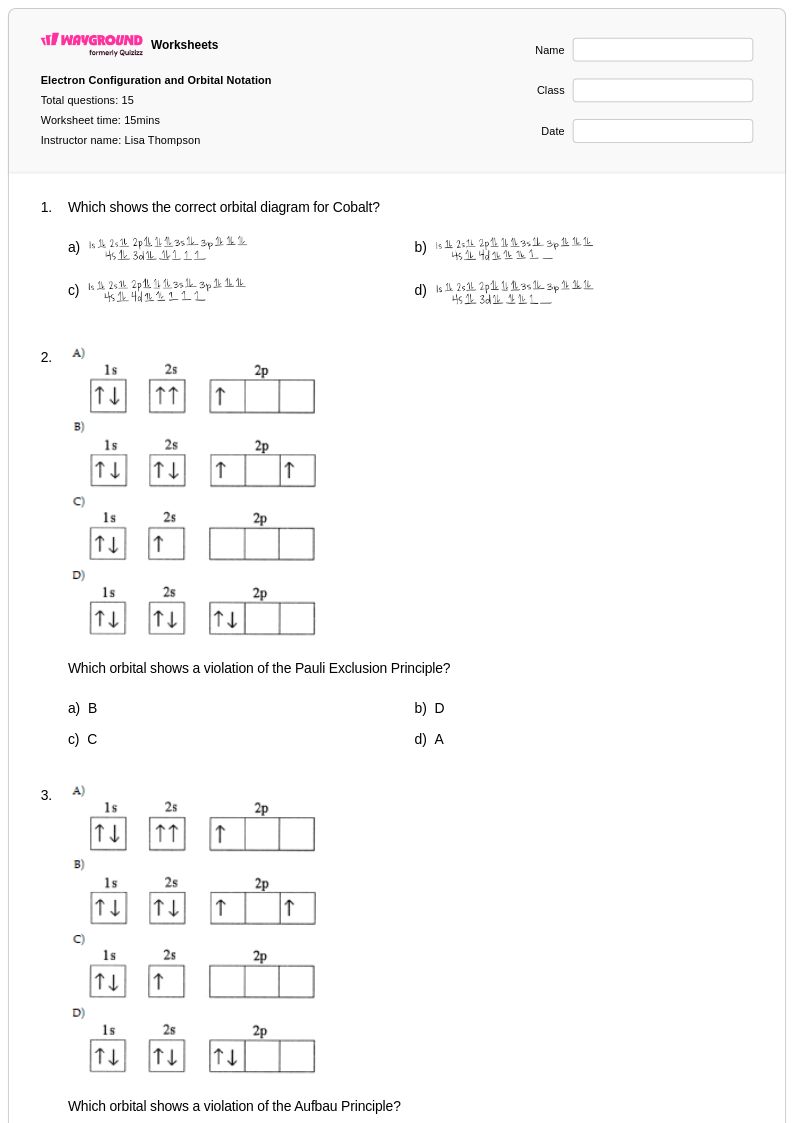
15 Q
11th - Uni

15 Q
11th - Uni

15 Q
11th - Uni

30 Q
12th

15 Q
11th - Uni
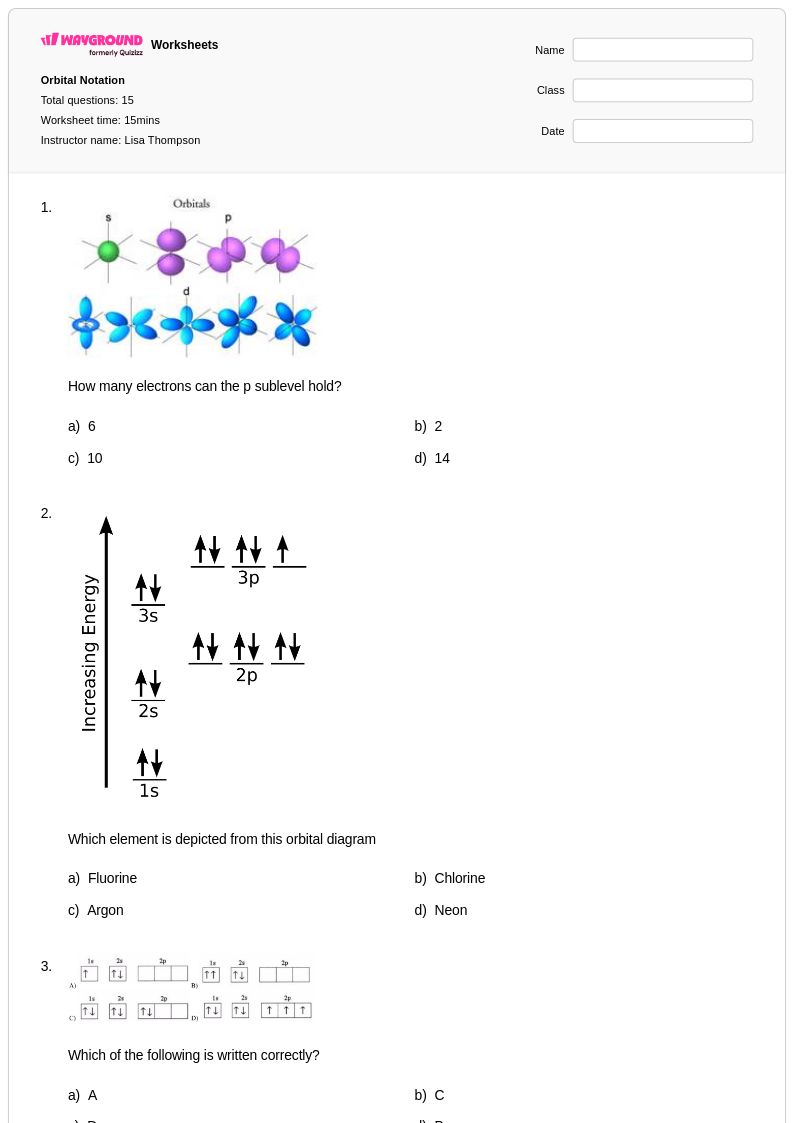
15 Q
11th - Uni
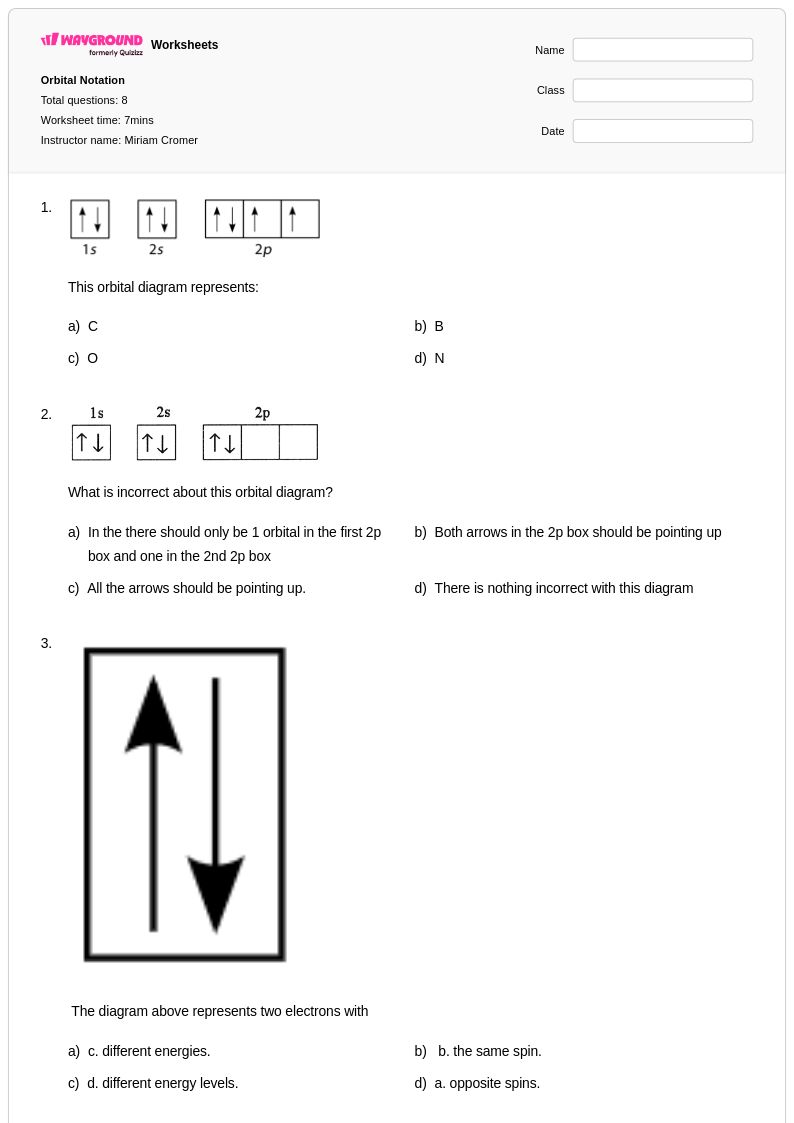
8 Q
9th - 12th

15 Q
11th - Uni
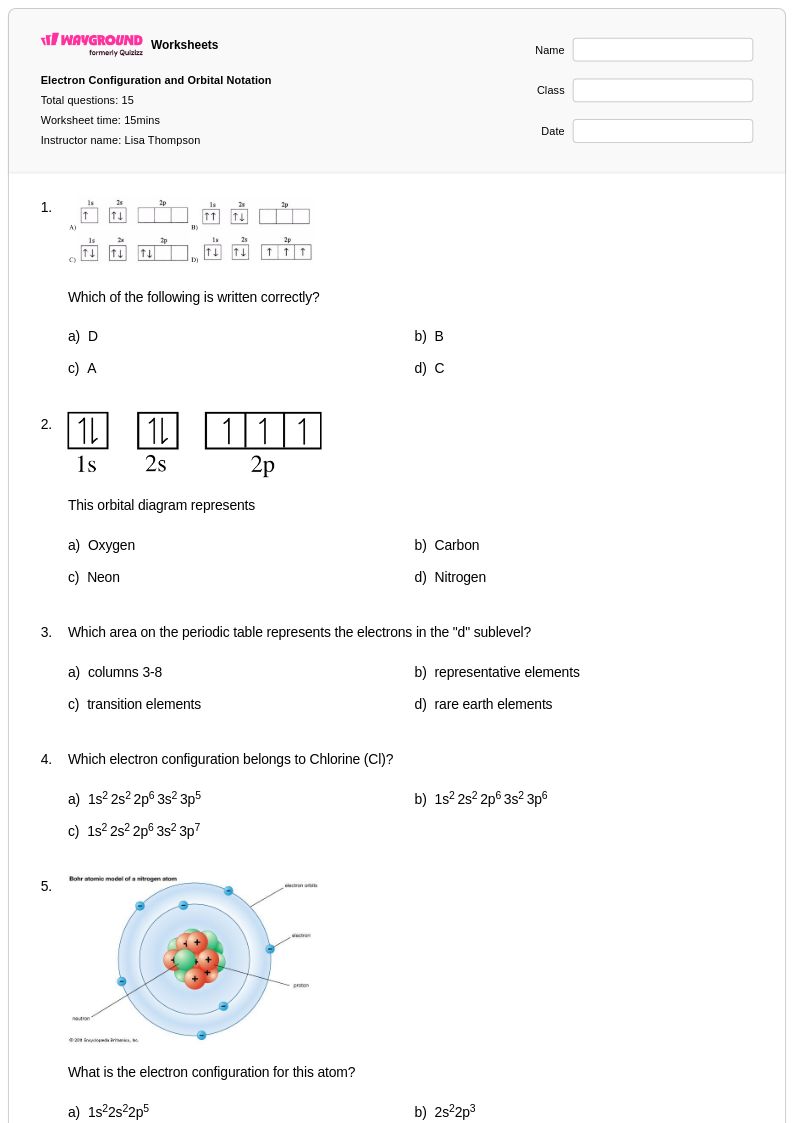
15 Q
11th - Uni
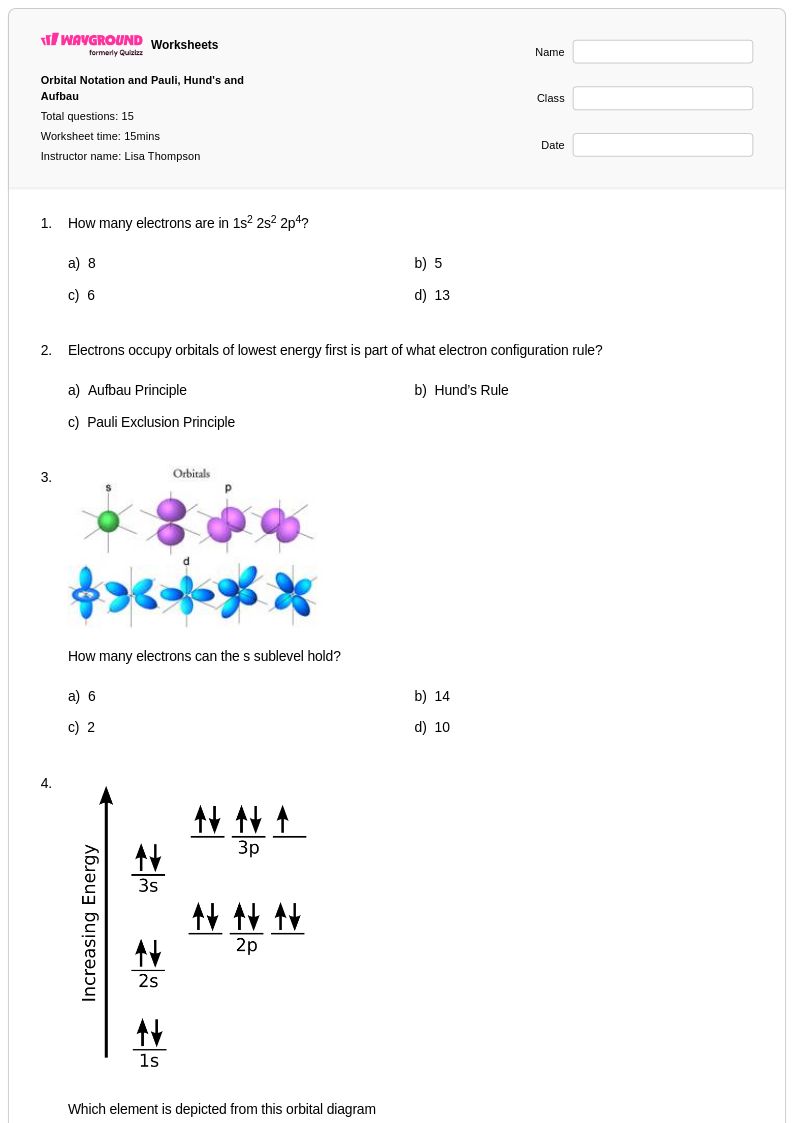
15 Q
11th - Uni

15 Q
11th - Uni
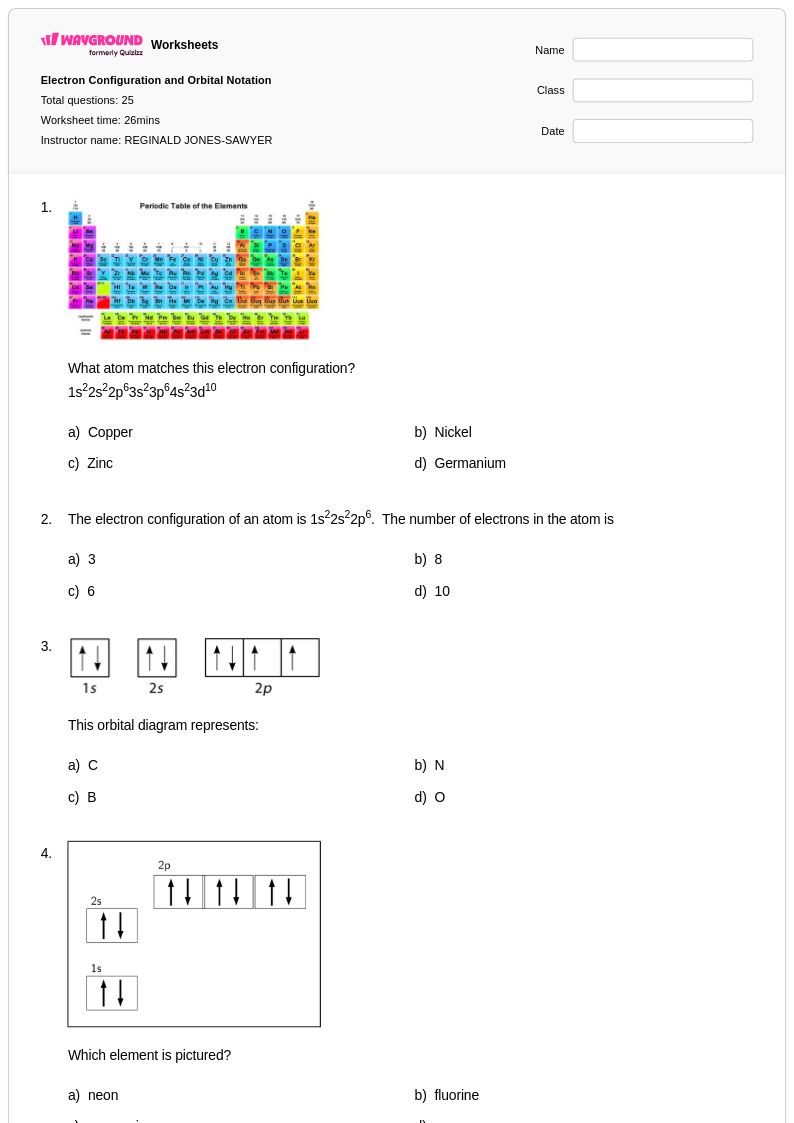
25 Q
9th - 12th
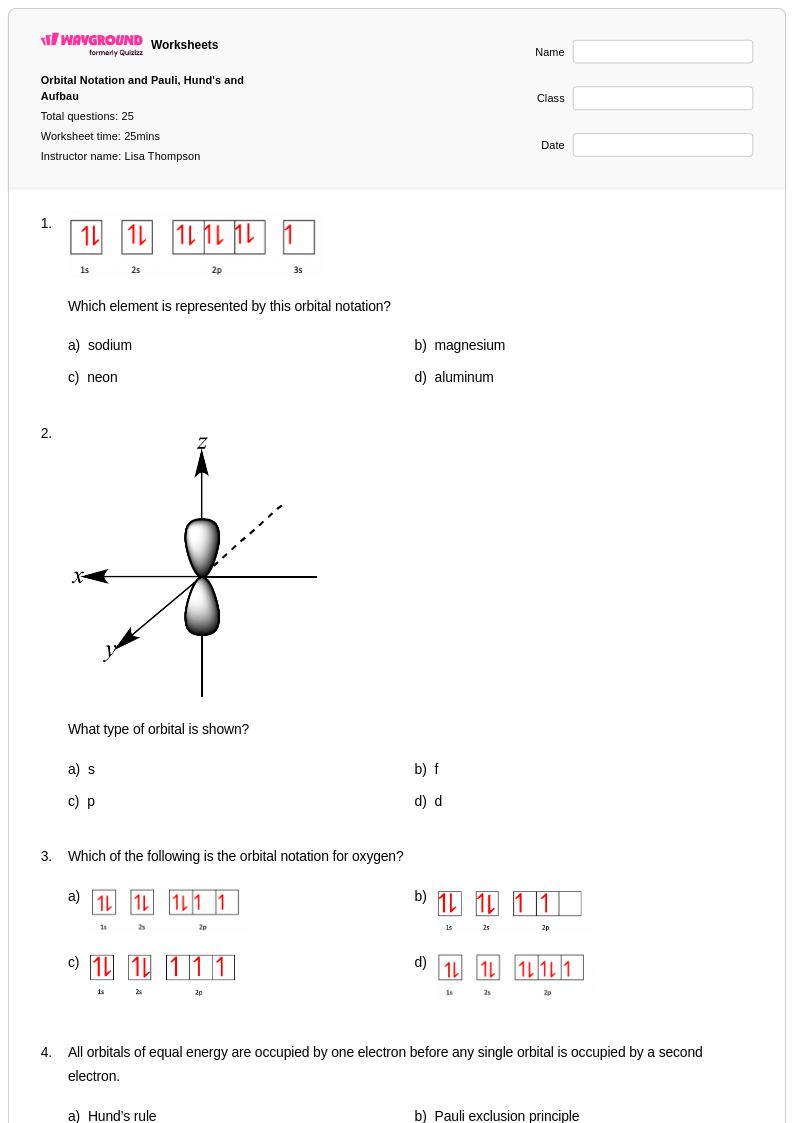
25 Q
11th - Uni
10 Q
9th - 12th

15 Q
10th - Uni
25 Q
11th - Uni

25 Q
10th - Uni

25 Q
10th - Uni

27 Q
12th
10 Q
9th - 12th
10 Q
9th - 12th
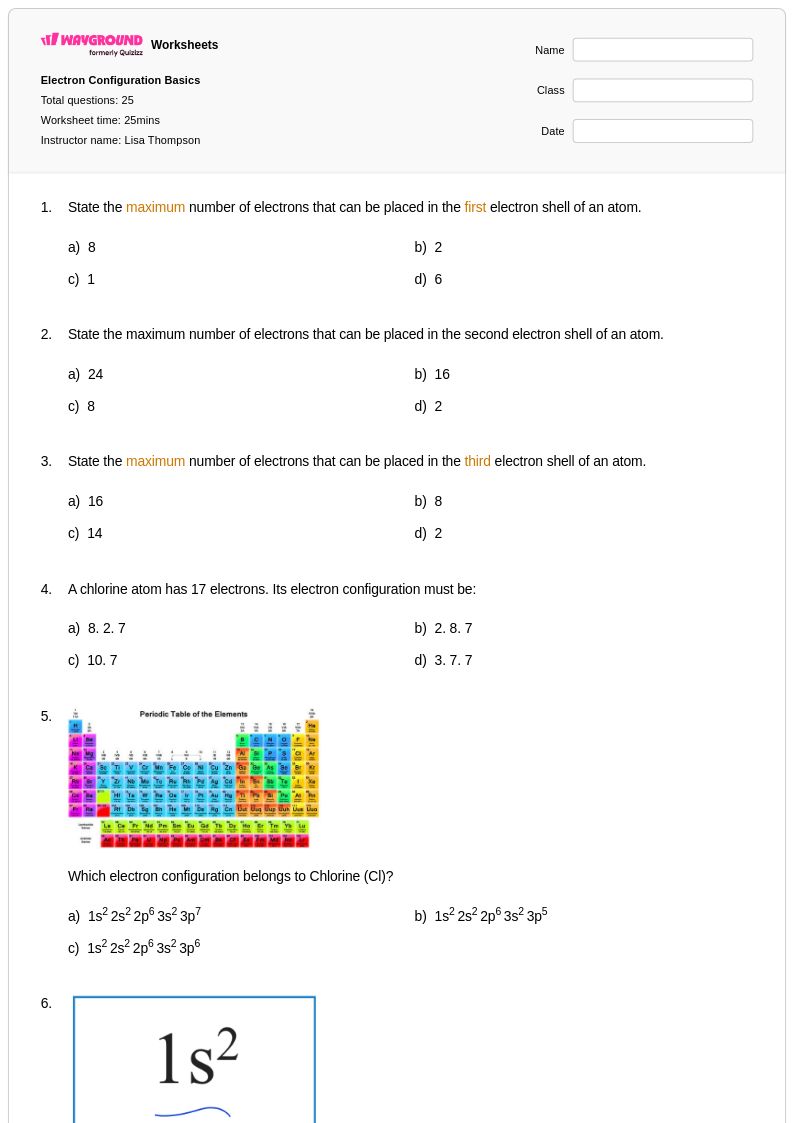
25 Q
10th - Uni
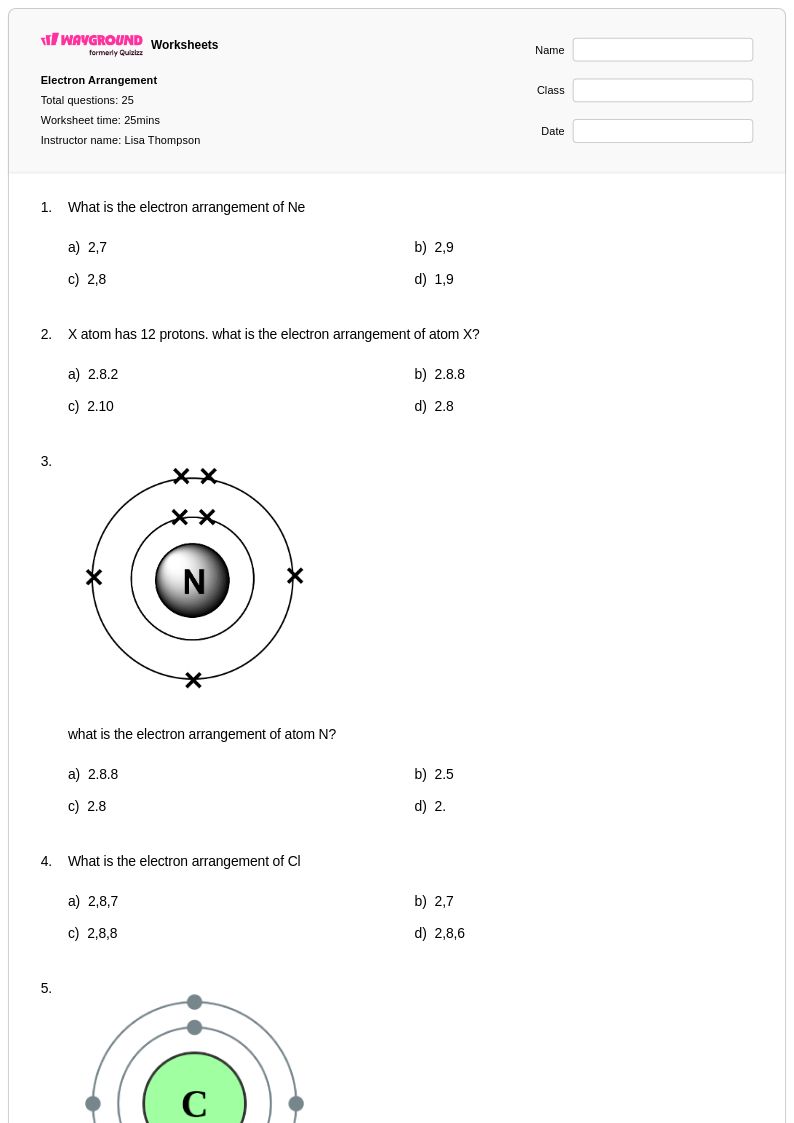
25 Q
8th - Uni
Explore Other Subject Worksheets for year 12
Explore printable Orbital Notation worksheets for Year 12
Orbital notation worksheets for Year 12 chemistry students available through Wayground (formerly Quizizz) provide comprehensive practice with electron configuration representations using boxes and arrows to show electron placement in atomic orbitals. These expertly designed worksheets strengthen students' understanding of quantum mechanical principles, helping them master the visualization of electron spin pairing, orbital filling order according to Hund's rule and the Aufbau principle, and the relationship between electron configuration and periodic trends. The collection includes practice problems ranging from simple main group elements to complex transition metals and ions, with complete answer keys and free printable pdf formats that allow students to work through increasingly challenging orbital diagrams while reinforcing their grasp of quantum numbers and electron behavior.
Wayground (formerly Quizizz) supports chemistry educators with millions of teacher-created orbital notation resources that can be easily searched and filtered by specific learning objectives, difficulty levels, and curriculum standards alignment. The platform's robust differentiation tools enable teachers to customize worksheets for diverse learning needs, offering both printable pdf versions for traditional assignments and digital formats for interactive practice sessions. These flexible resources facilitate targeted lesson planning, allowing educators to address common misconceptions about electron configuration, provide remediation for struggling students, and offer enrichment opportunities for advanced learners through progressively complex orbital notation challenges that build confidence in quantum mechanical concepts essential for success in advanced chemistry coursework.
