Recommended Topics for you
- Acid Base Nomenclature
- Acid Base Reactions
- Acid Base Theories
- Acid Base Titration
- Acids, Bases, and Salts
- Acids and Bases
- Activation Energy
- Activity Series
- Actual Yield
- Alcohol
- Alkanes Cycloalkanes and Functional Groups
- Alkene Nomenclature
- Alkenes and Alkynes
- Alkenes Naming
- Analyzing Substances
- Anions
- Aromatic Compounds
- Atom Structure
- Atomic Bonding
- Atomic Mass
- Atomic Mass Number
- Atomic Models
- Atomic Orbital Diagram
- Atomic Radius
- Atomic Structure
- Avogadro's Number
- Balancing Chemical Equations
- Balancing Nuclear Reactions
- Band of Stability
- Binary Ionic Compounds
- Bohr Model
- Bond Energy
- Bonding
- Building Atoms
- Burette Reading
- Calorimeter
- Calorimetry
- Cations
- Charles' Law
- Chemical Bonding
- Chemical Bonds
- Chemical Change
- Chemical Elements
- Chemical Equation Writing
- Chemical Equations
- Chemical Equilibrium
- Chemical Formulas
- Chemical Measurements
- Chemical Names and Formulas
- Chemical Naming
- Chemical Nomenclature
- Chemical Reactions
- Chemical Reactivity
- Chemistry Calculations
- Chemistry Lab Safety
- Chemistry Unit Conversions
- Classification of Chemical Reactions
- Classifying Reactions
- Colligative Properties
- Collision Theory
- Combined Gas Law
- Combustion Reaction
- Composition
- Concentration of Solutions
- Concentrations
- Conjugate Acid Base
- Conjugate Acid Base Pairs
- Conservation of Mass
- Conservation of Matter
- Conversion Factors in Chemistry
- Conversion Problems
- Coulombic Attraction
- Counting Atoms
- Counting Atoms in Chemical Formulas
- Counting Atoms in Compounds
- Covalent Bond
- Covalent Bonding
- Covalent Compounds
- Dalton's Law
- Decay
- Dilutions
- Dipole Moments
- Double Displacement
- Double Displacement Reaction
- Double Replacement Reaction
- Electrochemical Cell
- Electrolytes
- Electron Arrangements
- Electron Configuration
- Electron Dot Diagram
- Electron Orbitals
- Electronegativity
- Electronic Structure of Atoms
- Electrons
- Element Symbols
- Empirical and Molecular Formula
- Endothermic and Exothermic Processes
- Energy in Reactions
- Energy Levels
- Enthalpy
- Enthalpy of Formation
- Enthalpy Stoichiometry
- Fertilizer Calculation
- Flame Test
- Formal Charge
- Forming Ions
- Formula Mass
- Formula Writing
- Functional Group Identification
- Galvanic Cell
- Gas Laws
- Gas Properties
- Gas Variables
- Graham's Law of Effusion
- Heat of Formation
- Heat of Fusion
- Heat of Vaporization
- Heat Transfer and Thermal Equilibrium
- Heating Curve
- Hess's Law
- History of Atomic Models
- History of Chemistry
- Homogeneous and Heterogeneous Mixtures
- Hydrocarbons
- Ideal Gas Equation
- Ideal Gas Law
- Identifying Reactions
- Infrared Spectroscopy
- Intermolecular Forces
- Interpreting Spectra
- Ion Formation
- Ionic and Covalent Bonding
- Ionic and Covalent Compounds
- Ionic Bonding
- Ionic Bonds
- Ionic Charges
- Ionic Compound Formula Writing
- Ionic Compound Formulas
- Ionic Compounds
- Ionic Formula Writing
- Ionic Formulas
- Ionization Energy
- Ions
- Ions and Atoms
- Ions and Formula Units
- Ions and Isotopes
- IR Spectroscopy
- Isotope
- Isotope Notation
- Iupac Naming
- Lab Equipment
- Laboratory Equipment
- Law of Conservation of Mass
- Le Chatelier's Principle
- Lewis Dot Diagram
- Lewis Dot Structure
- Lewis Structure
- Limiting Reactants
- Limiting Reagent
- Mass and Moles
- Mass Relationships in Chemical Reactions
- Mass Spectrometry
- Mass Spectroscopy
- Metallic Bonding
- Metalloids
- Metals
- Models of Atoms
- Molality
- Molarity
- Molarity and Dilution
- Mole and Volume
- Mole Concept
- Mole Conversions
- Mole Gram Conversion
- Mole Ratios
- Mole Relationships
- Mole to Mass Conversion
- Molecular Geometry
- Molecular Mass
- Molecular Shapes
- Molecular Structure
- Molecule
- Moles
- Naming Acids
- Naming Alkanes
- Naming Binary Compounds
- Naming Binary Ionic Compounds
- Naming Binary Molecular Compounds
- Naming Chemical Compounds
- Naming Compounds
- Naming Covalent Compounds
- Naming Hydrocarbons
- Naming Ionic and Covalent Bonds
- Naming Ionic and Covalent Compounds
- Naming Ionic Compounds
- Naming Molecular Compounds
- Naming Organic Compounds
- Net Ionic Equation
- Neutralisation Reaction
- Neutralization
- Neutralization Reactions
- Nomenclature
- Nomenclature for Ionic Compounds
- Nomenclature of Acids
- Nomenclature of Alkanes
- Nonmetals
- Nuclear Changes
- Nuclear Chemistry
- Nuclear Equations
- Orbital Diagrams
- Orbital Notation
- Orbitals
- Organic Chemistry
- Organic Molecules
- Oxidation
- Oxidation Numbers
- Oxidation Reduction Reactions
- Oxidation State
- Percent Abundance of Isotopes
- Percent Composition by Mass
- Percent Yield
- Percentage Composition
- Percentage Yield
- Periodic Table
- Periodic Table Organization
- Periodic Table Trends
- Periodic Trends
- PH and POH Calculations
- PH Scale
- Phase Change
- Phase Diagram
- Physical and Chemical Properties and Changes
- Physical Properties
- Polar and Nonpolar Molecules
- Polar Molecules
- Polarity
- Polarity of Bonds
- Polarity of Molecules
- Polyatomic Ions
- Polymers
- Precipitation Reaction
- Predicting Products of Chemical Reactions
- Properties of Water
- Quantum Numbers
- Rate Law Expression
- Rate Laws
- Reaction Order
- Reaction Rates
- Redox Equations
- Redox Reactions and Electrochemistry
- Relative Mass
- Resonance Structure
- Rutherford Gold Foil Experiment
- Salts
- Separation Methods
- Separation Techniques
- Single and Double Replacement Reactions
- Single Displacement Reaction
- Single Replacement Reaction
- Solubility
- Solubility Curves
- Solubility Rules
- Standard Heats of Formation
- Stereochemistry of Alkene Additions
- Stoichiometric Calculations
- Stoichiometry
- Stoichiometry of Gases
- Theoretical Yield
- Thermochemistry
- Thin Layer Chromatography
- Timeline
- Titration
- Titration Calculations
- Titration Curves
- Types of Chemical Reactions
- Types of Reactions
- Valence Electrons
- Voltaic Cell
- VSEPR Theory
- Word Equations
- Writing Chemical Formulas for Ionic Compounds
- Writing Formulas for Covalent Compounds

18 Q
10th - Uni
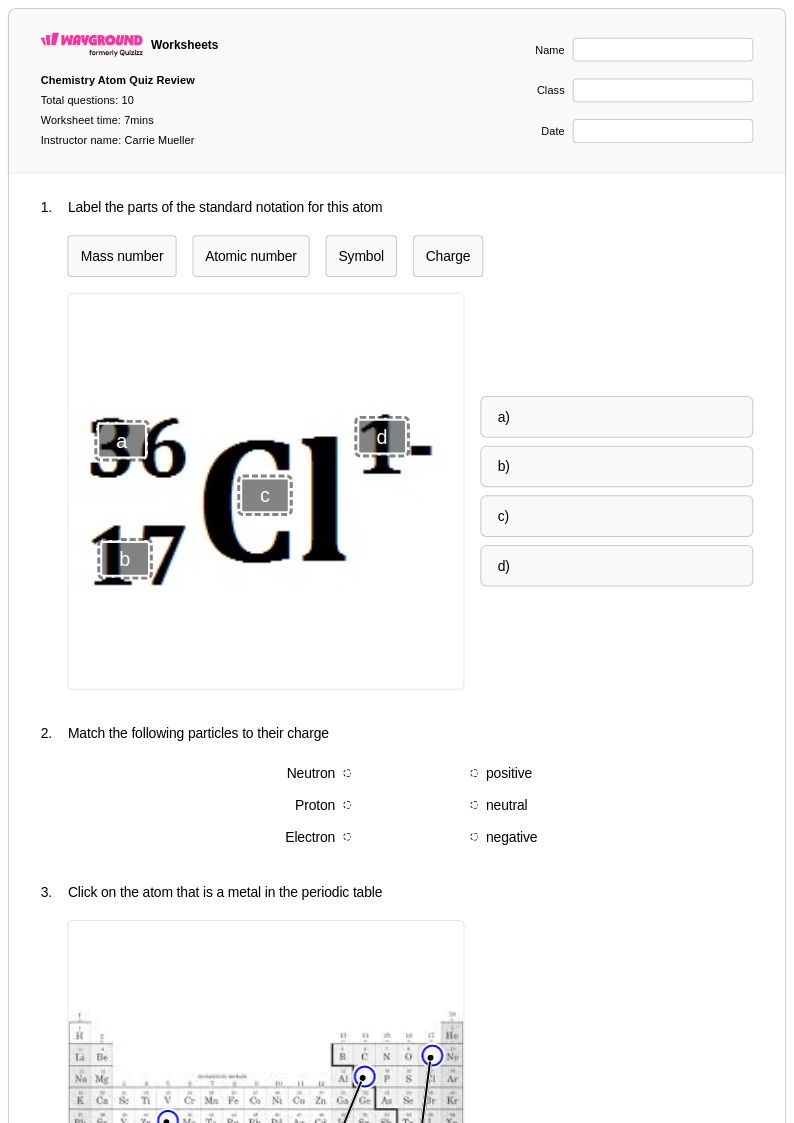
10 Q
10th
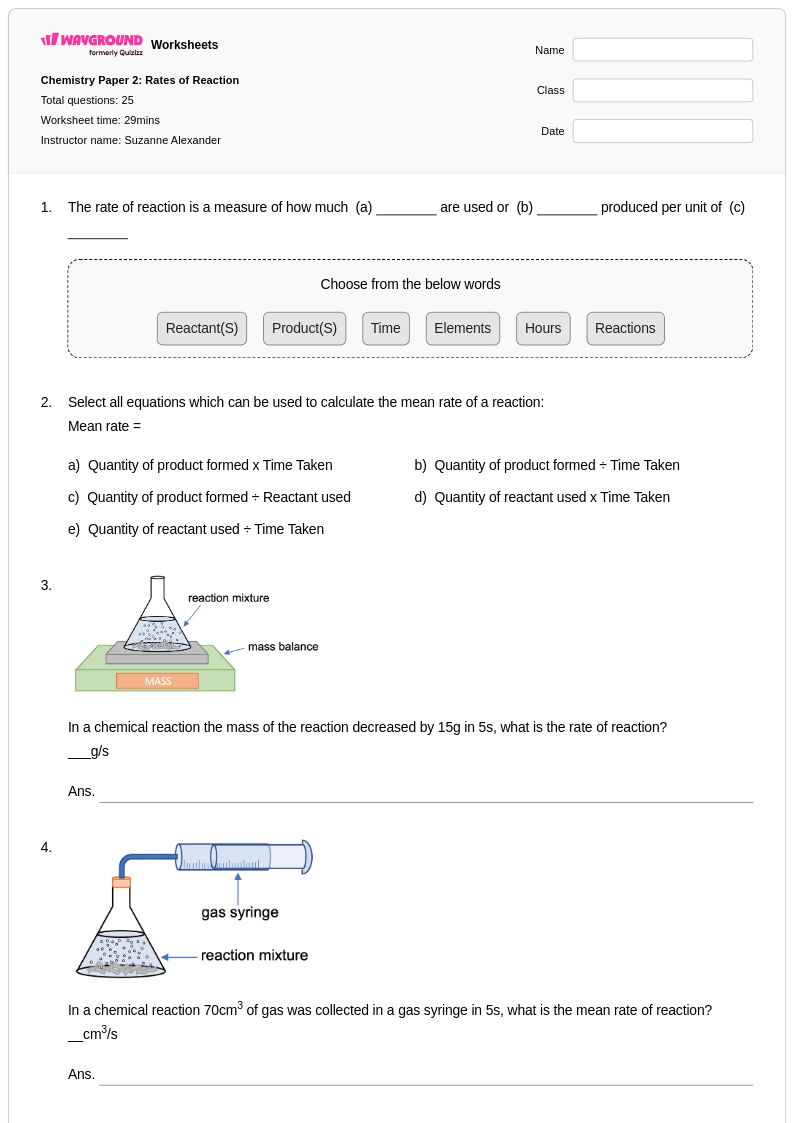
25 Q
9th - 12th
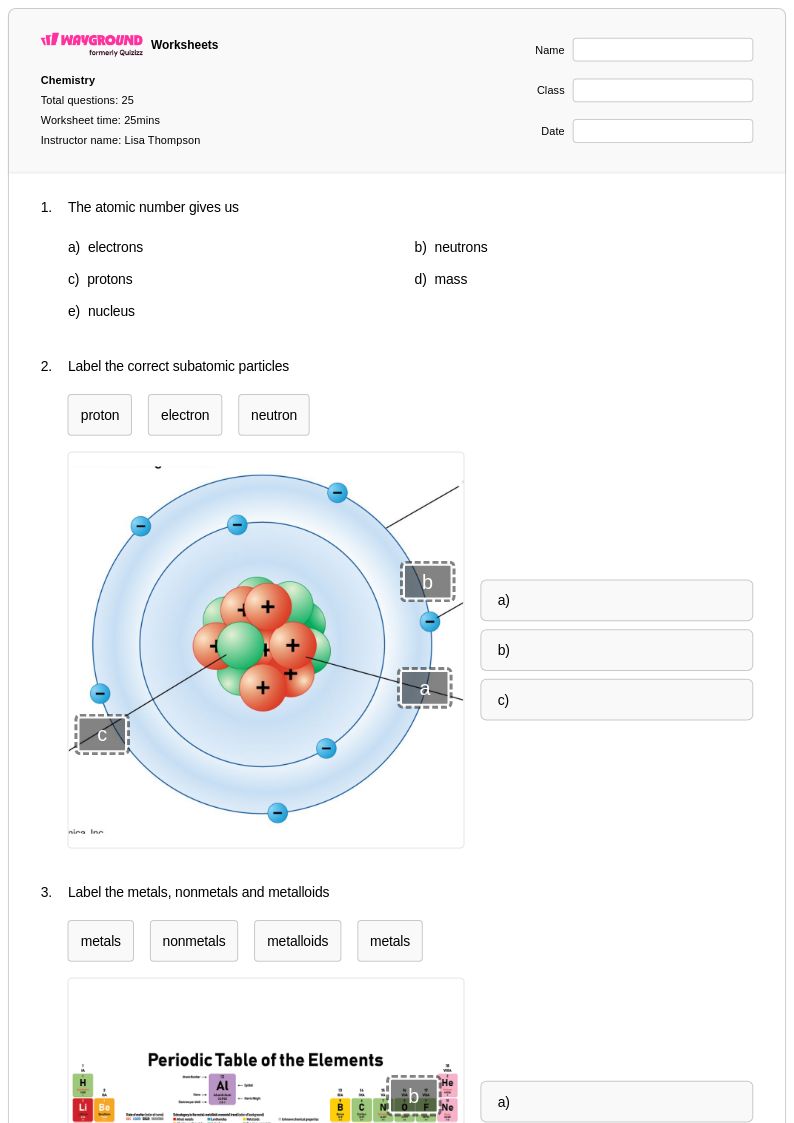
25 Q
7th - Uni
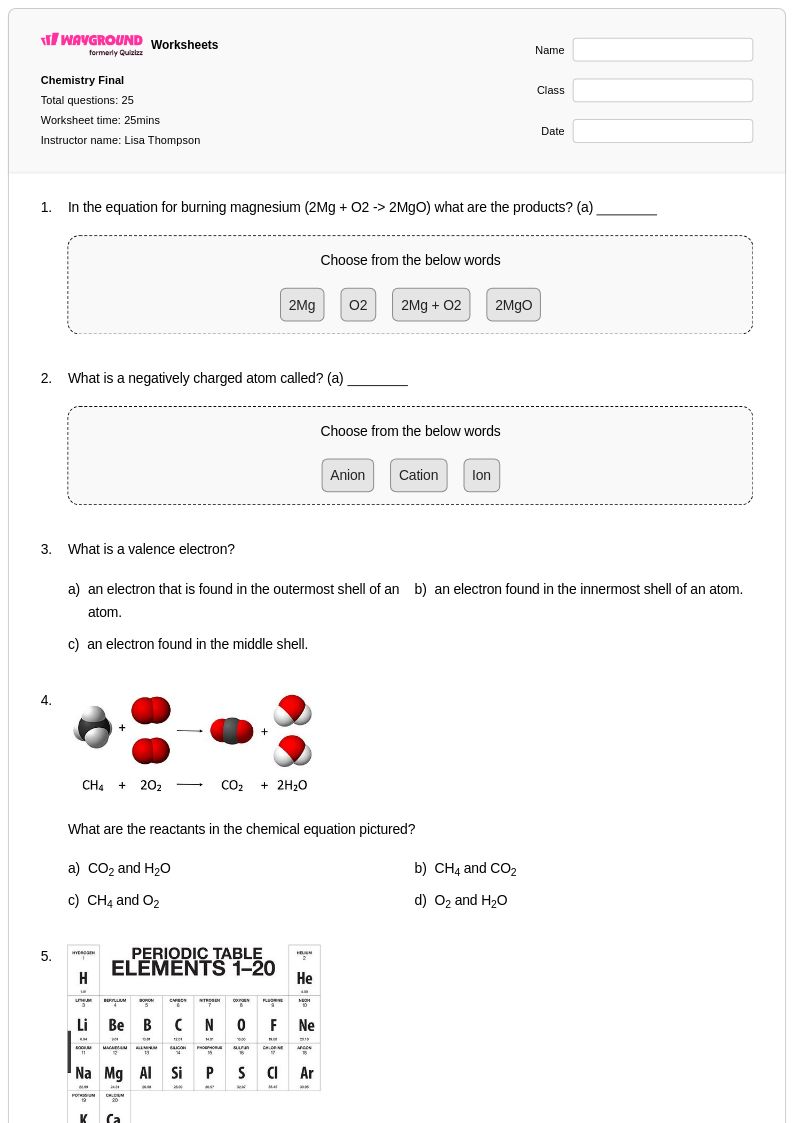
25 Q
8th - Uni
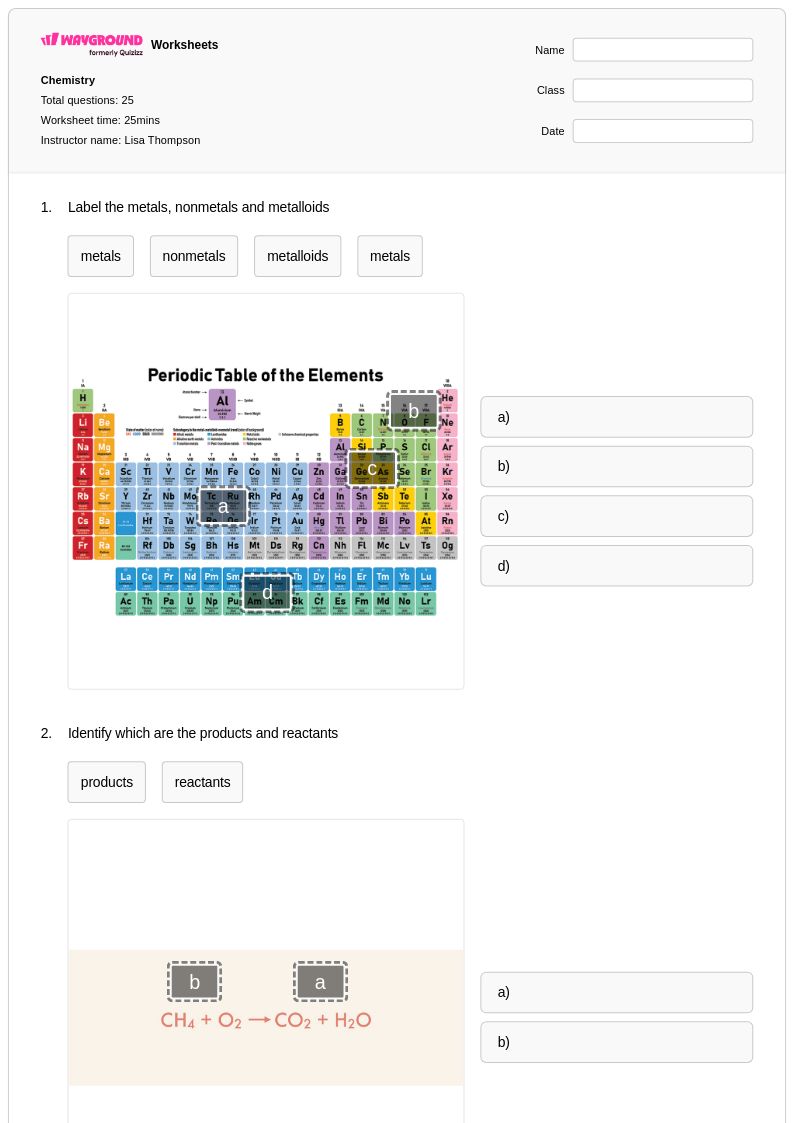
25 Q
8th - Uni

29 Q
9th - 12th
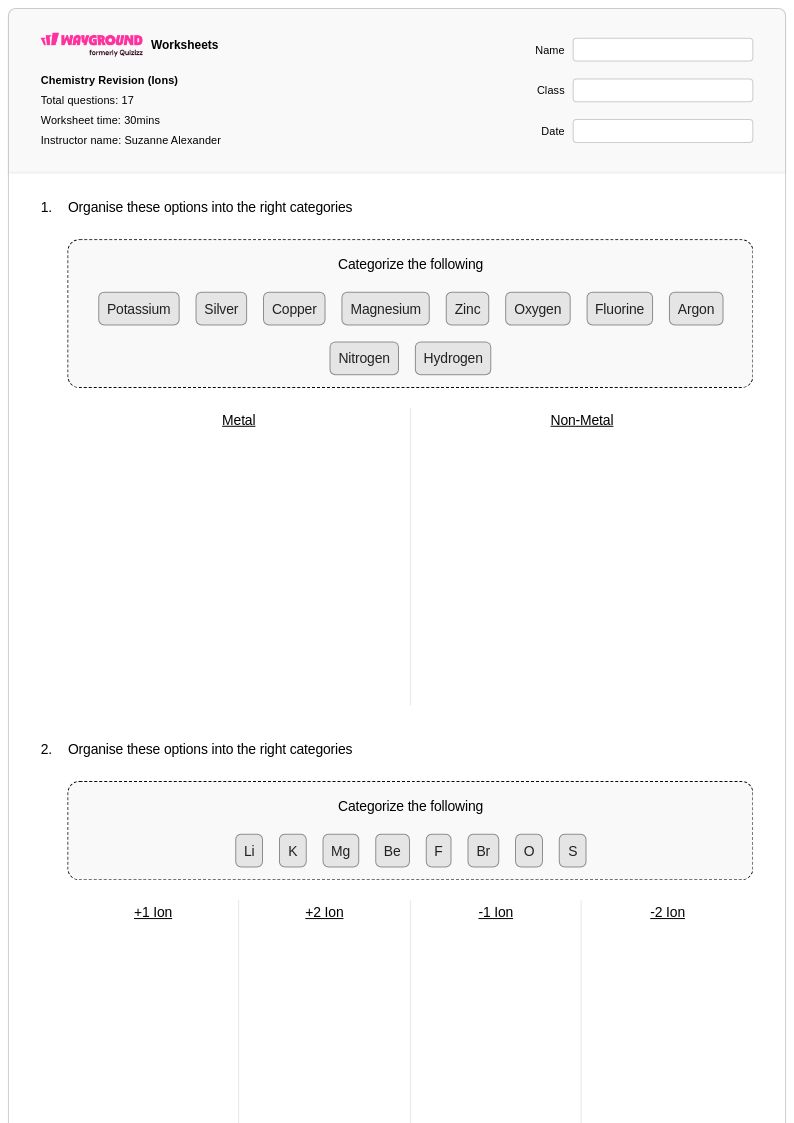
17 Q
9th - 12th

25 Q
8th - Uni

50 Q
10th

15 Q
8th - Uni

15 Q
8th - Uni

15 Q
8th - Uni

15 Q
8th - Uni
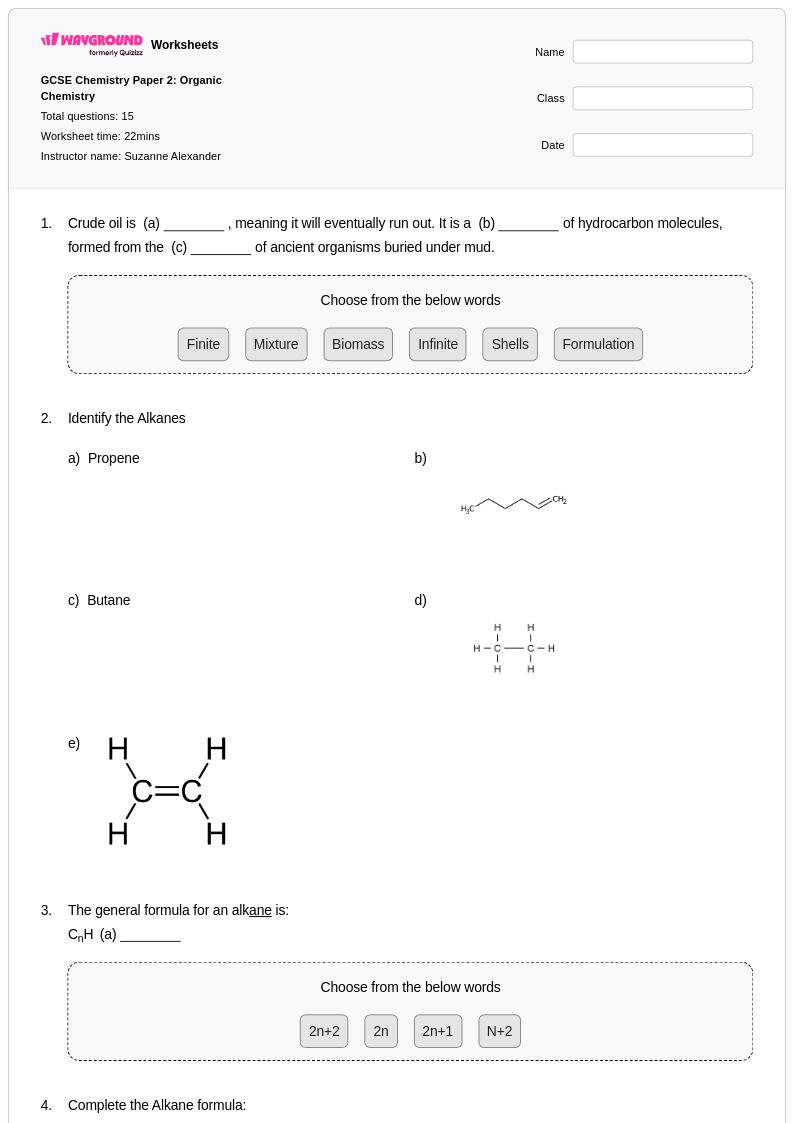
15 Q
9th - 12th

84 Q
10th

25 Q
6th - Uni
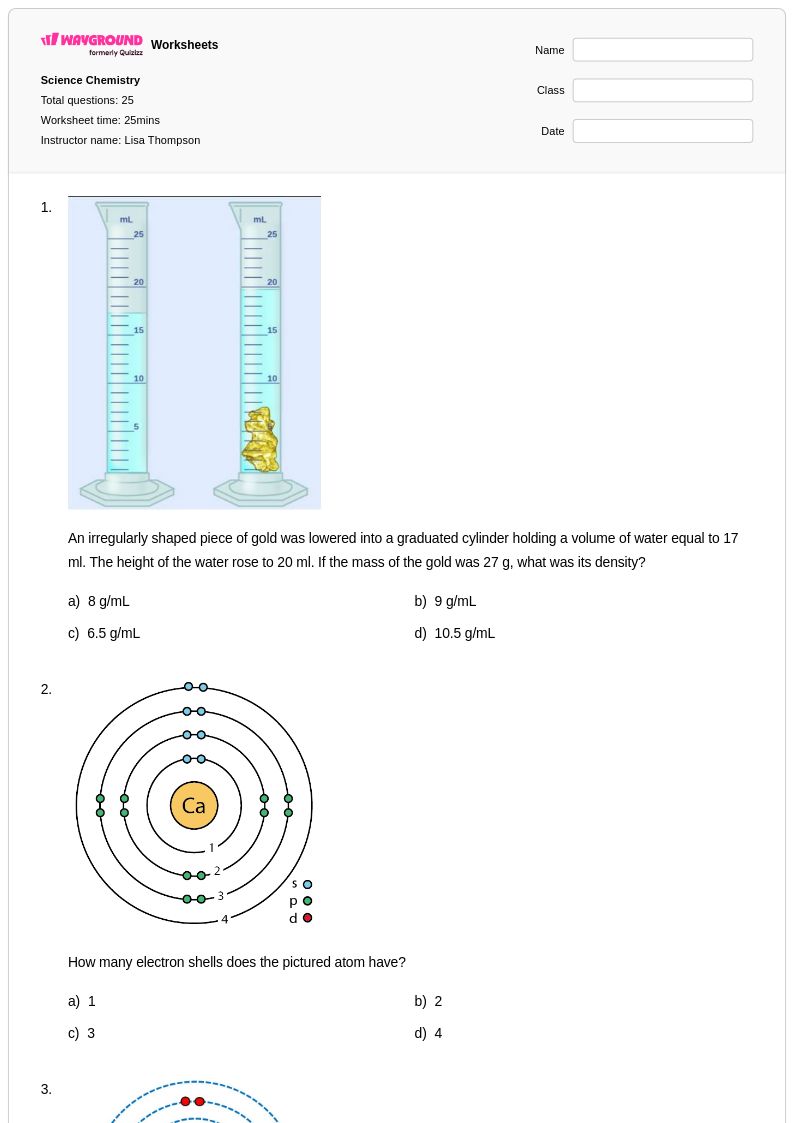
25 Q
8th - Uni

25 Q
8th - Uni

15 Q
8th - Uni

15 Q
10th - Uni

15 Q
10th - Uni

15 Q
10th - Uni
15 Q
8th - Uni
Explore Chemistry Worksheets by Grades
Explore Chemistry Worksheets for class 10 by Topic
- Acid Base Nomenclature
- Acid Base Reactions
- Acid Base Theories
- Acid Base Titration
- Acids, Bases, and Salts
- Acids and Bases
- Activation Energy
- Activity Series
- Actual Yield
- Alcohol
- Alkanes Cycloalkanes and Functional Groups
- Alkene Nomenclature
- Alkenes and Alkynes
- Alkenes Naming
- Analyzing Substances
- Anions
- Aromatic Compounds
- Atom Structure
- Atomic Bonding
- Atomic Mass
Explore Other Subject Worksheets for class 10
Explore printable Chemistry worksheets for Class 10
Class 10 chemistry worksheets available through Wayground (formerly Quizizz) provide comprehensive coverage of fundamental chemical concepts that form the foundation of advanced scientific study. These expertly crafted resources strengthen students' understanding of atomic structure, chemical bonding, stoichiometry, and reaction mechanisms through systematic practice problems that progress from basic applications to complex multi-step calculations. Each worksheet collection includes detailed answer keys that support independent learning and self-assessment, while the printable pdf format ensures accessibility whether students are working in traditional classrooms or remote learning environments. The free practice problems encompass essential topics such as periodic trends, molecular geometry, thermodynamics, and acid-base chemistry, allowing students to develop the analytical thinking skills and mathematical precision required for success in advanced chemistry courses.
Wayground's extensive library of teacher-created chemistry worksheets supports educators with millions of high-quality resources specifically designed for Class 10 learners. The platform's robust search and filtering capabilities enable teachers to quickly locate materials aligned with specific curriculum standards, whether they need targeted practice for electron configuration, balanced equation worksheets, or comprehensive unit reviews. Differentiation tools allow instructors to customize content difficulty levels and modify problem sets to meet diverse learning needs, making these resources invaluable for both remediation and enrichment activities. The flexibility of accessing materials in both printable and digital formats, including downloadable pdfs, streamlines lesson planning while providing options for immediate classroom use, homework assignments, or assessment preparation that adapts to any instructional environment.
