8 Q
11th - 12th

15 Q
11th - Uni
20 Q
11th - 12th
25 Q
10th - Uni

15 Q
11th - Uni

15 Q
11th - Uni
8 Q
9th - 12th

25 Q
10th - Uni

25 Q
11th - Uni
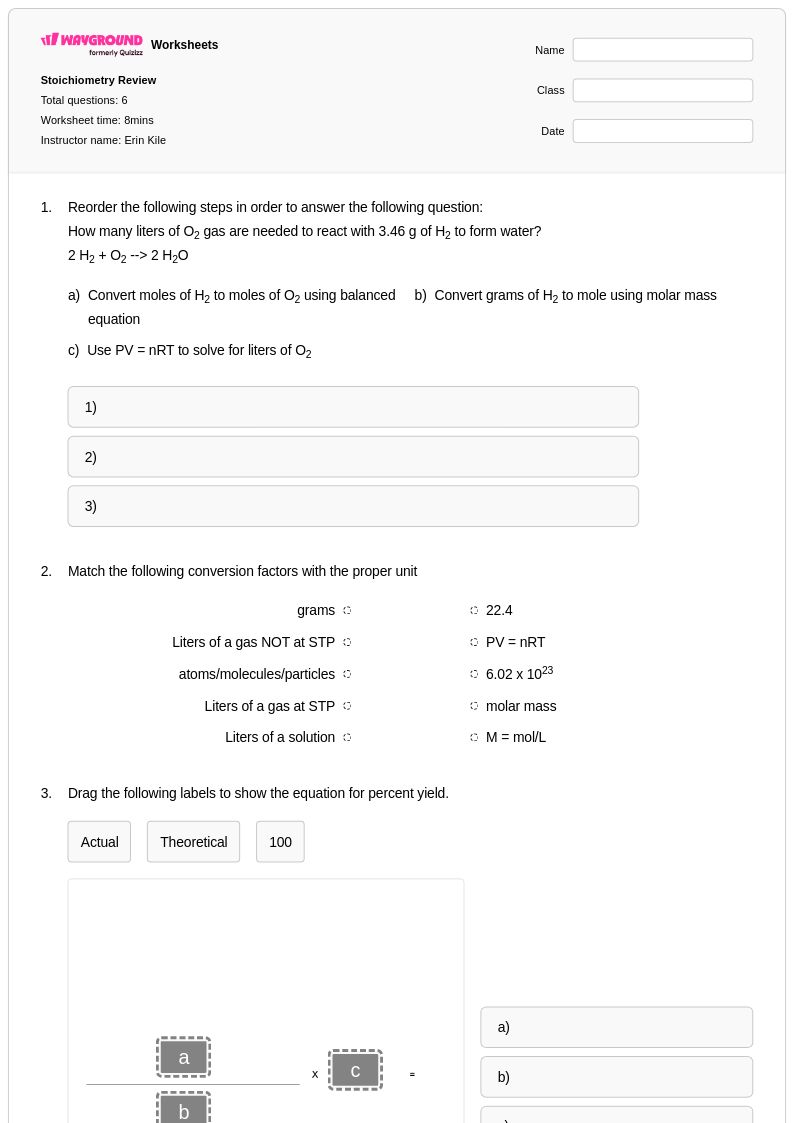
6 Q
9th - 12th

15 Q
9th - 12th

22 Q
11th
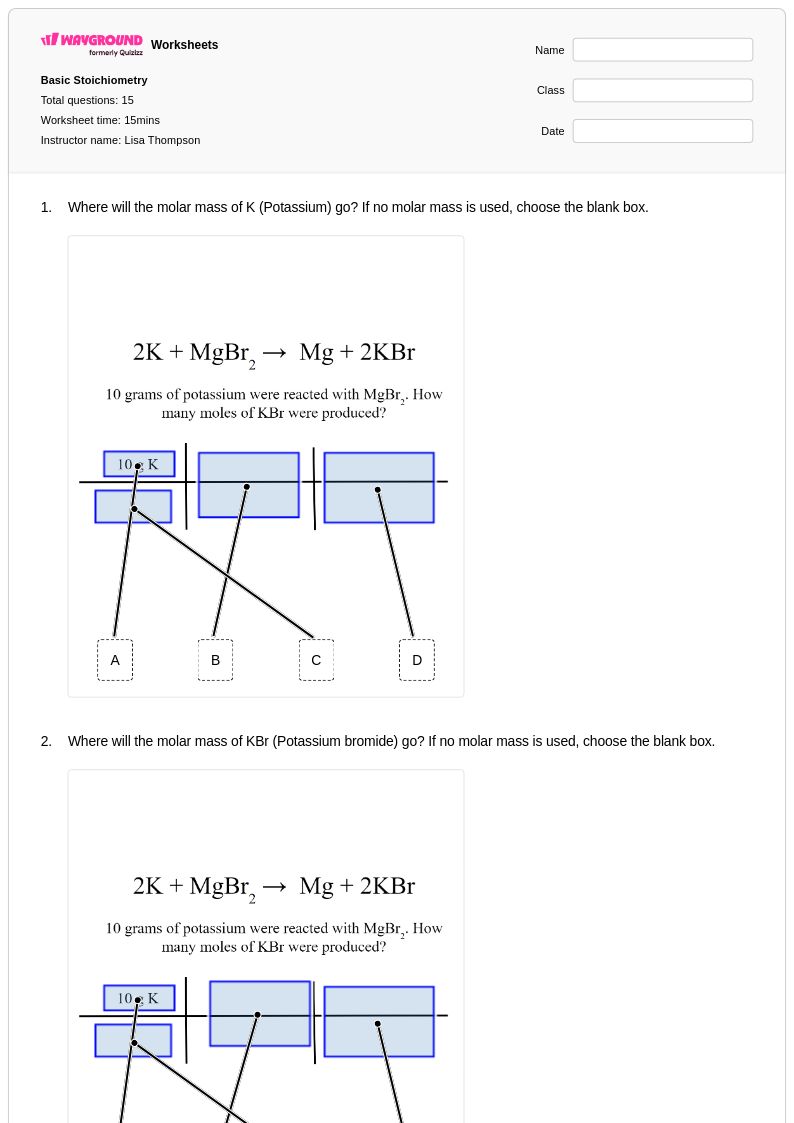
15 Q
10th - Uni

15 Q
11th - Uni

15 Q
10th - Uni
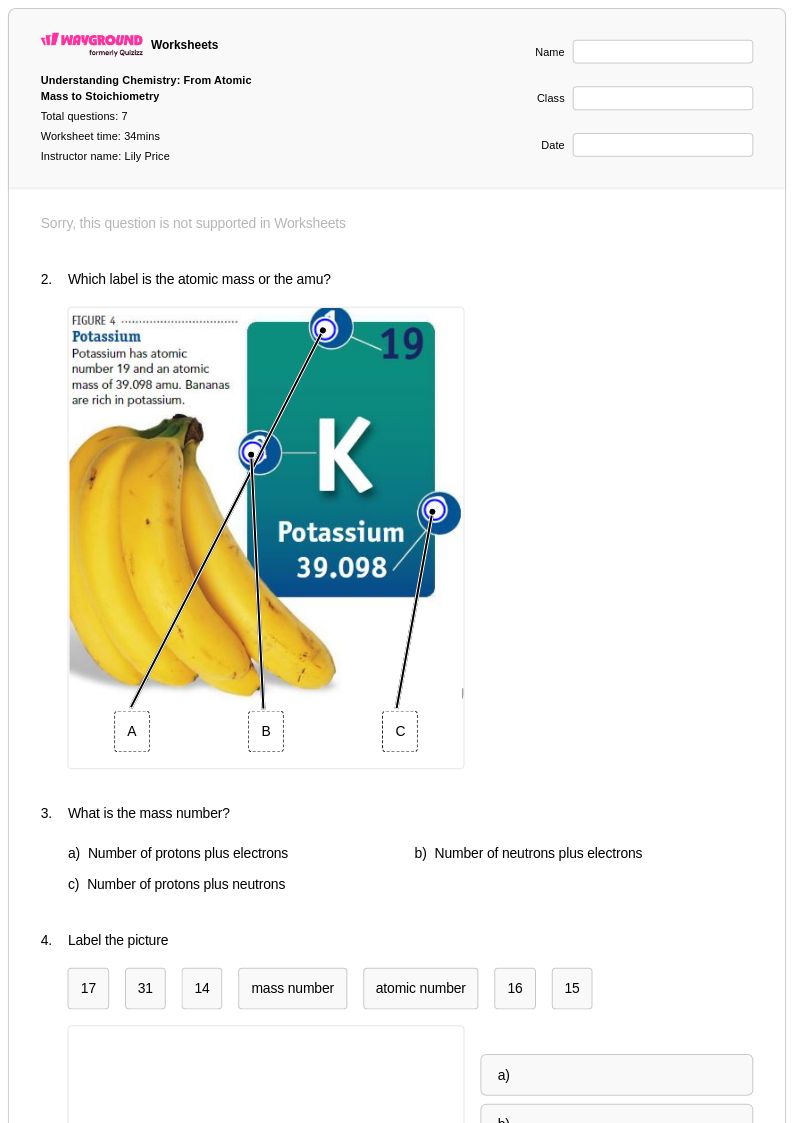
17 Q
9th - 12th
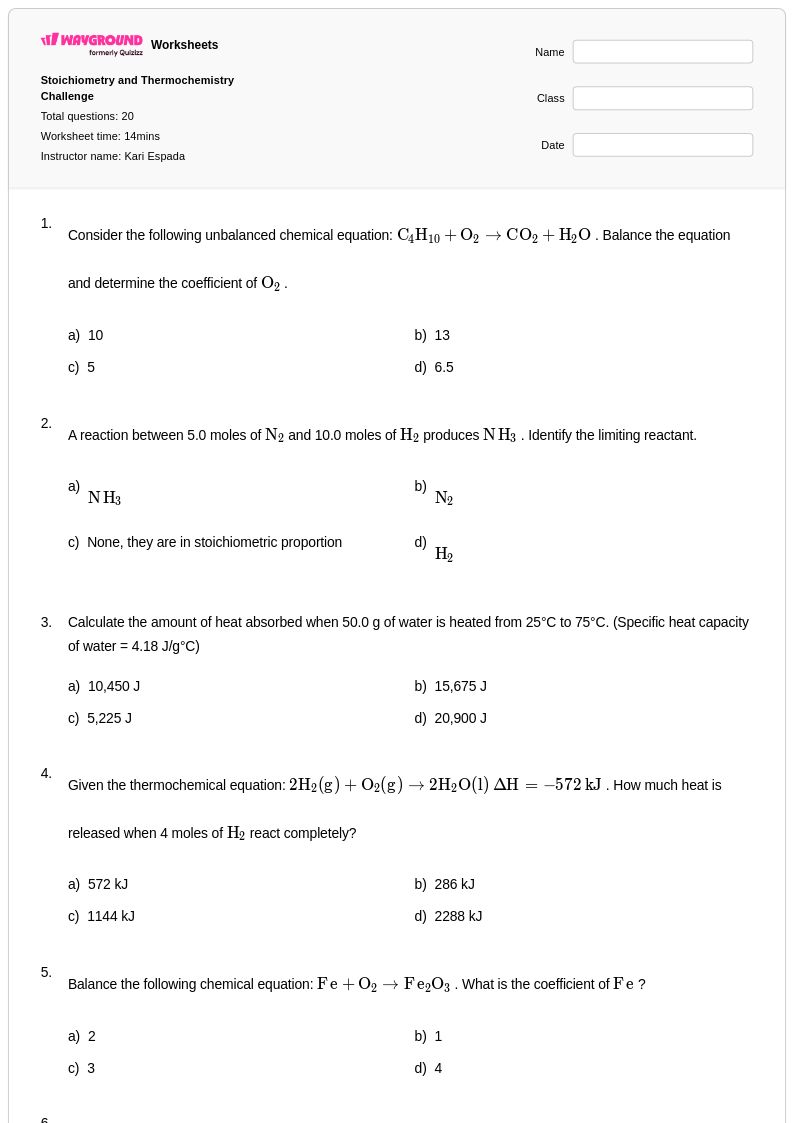
20 Q
11th

74 Q
9th - 12th

25 Q
11th - Uni

15 Q
10th - Uni

15 Q
10th - Uni

28 Q
11th
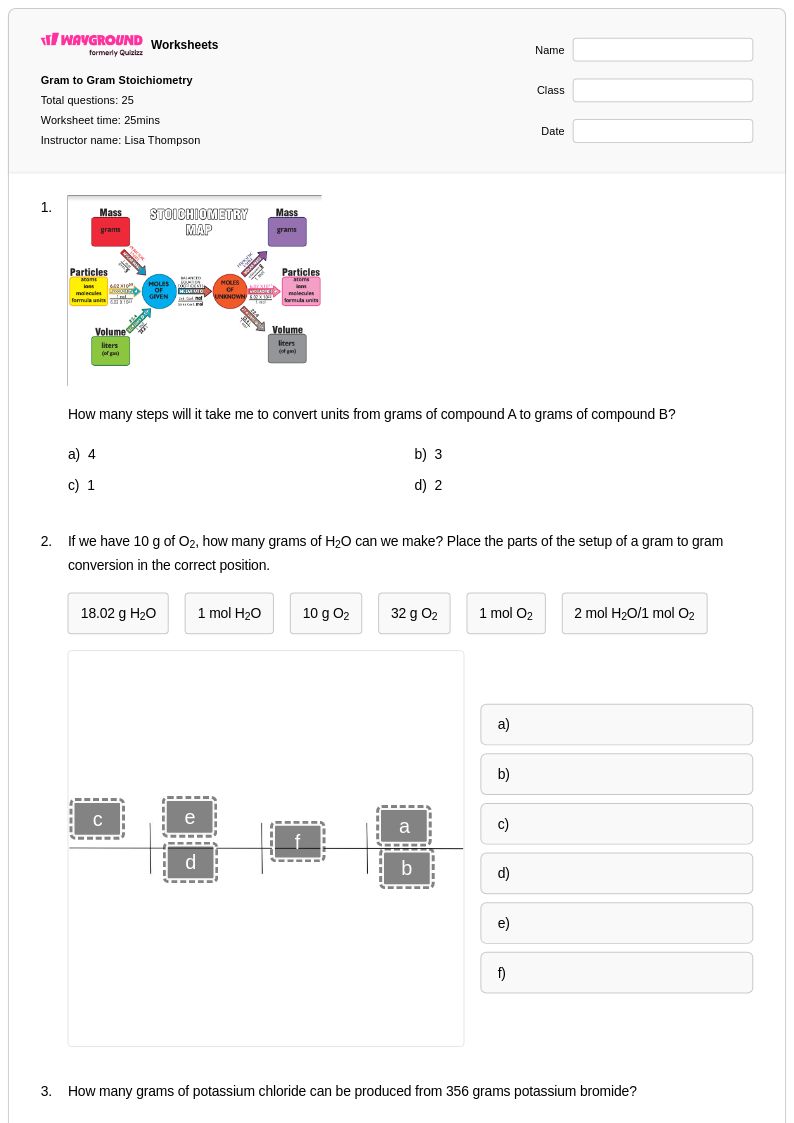
25 Q
10th - Uni
25 Q
11th - Uni
Explore Other Subject Worksheets for year 11
Explore printable Enthalpy Stoichiometry worksheets for Year 11
Enthalpy stoichiometry worksheets for Year 11 students available through Wayground (formerly Quizizz) provide comprehensive practice with the quantitative relationships between chemical reactions and energy changes. These educational resources strengthen students' ability to calculate enthalpy changes using balanced chemical equations, apply Hess's law to multi-step reactions, and determine the energy requirements for various chemical processes. The worksheets feature systematic practice problems that guide students through complex calculations involving standard enthalpies of formation, combustion reactions, and calorimetry data. Each printable resource includes detailed answer keys that help students verify their work and understand common problem-solving approaches, while the free pdf format ensures easy classroom distribution and home study access.
Wayground (formerly Quizizz) supports chemistry educators with millions of teacher-created enthalpy stoichiometry resources that streamline lesson planning and student assessment. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific curriculum standards and learning objectives, while built-in differentiation tools enable customization for varying skill levels within Year 11 classrooms. Teachers can access these materials in both printable and digital formats, including downloadable pdf versions that facilitate hybrid learning environments. These extensive worksheet collections prove invaluable for targeted remediation of struggling students, enrichment activities for advanced learners, and systematic skill practice that reinforces the connection between thermochemistry concepts and stoichiometric calculations throughout the academic year.
