
55 Q
11th

15 Q
10th - Uni

12 Q
11th

14 Q
9th - 12th
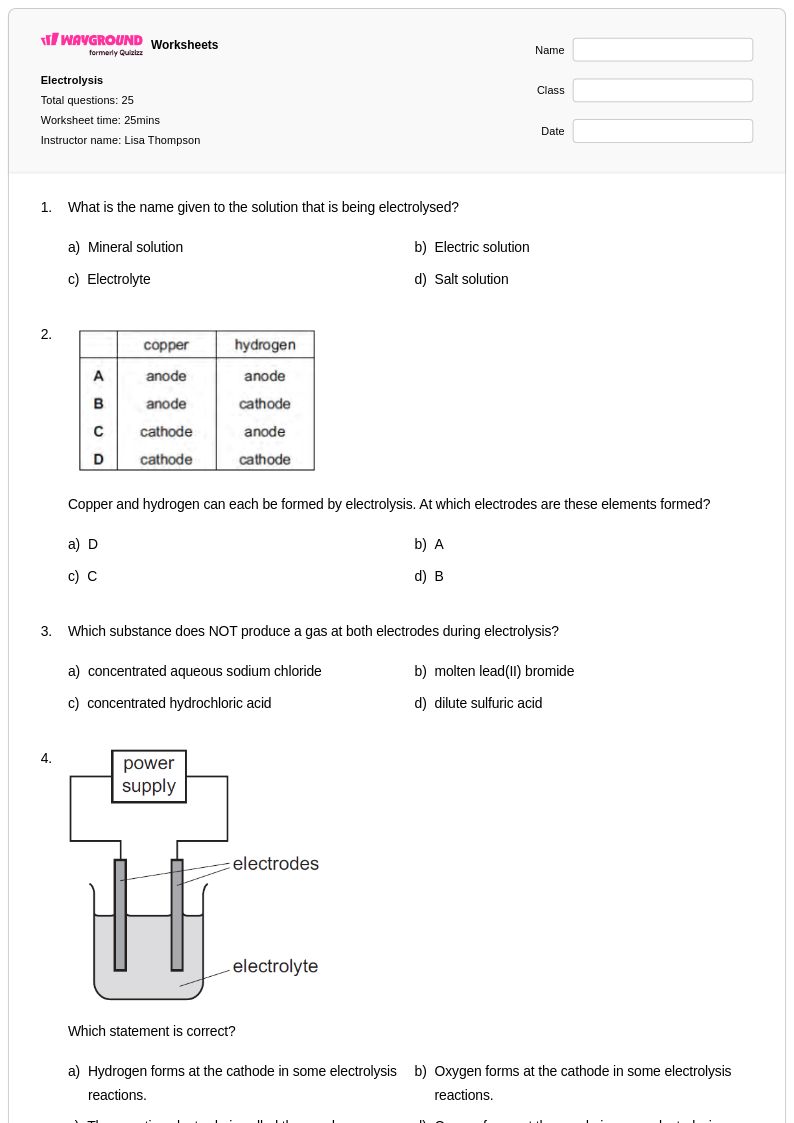
25 Q
10th - Uni
11 Q
11th
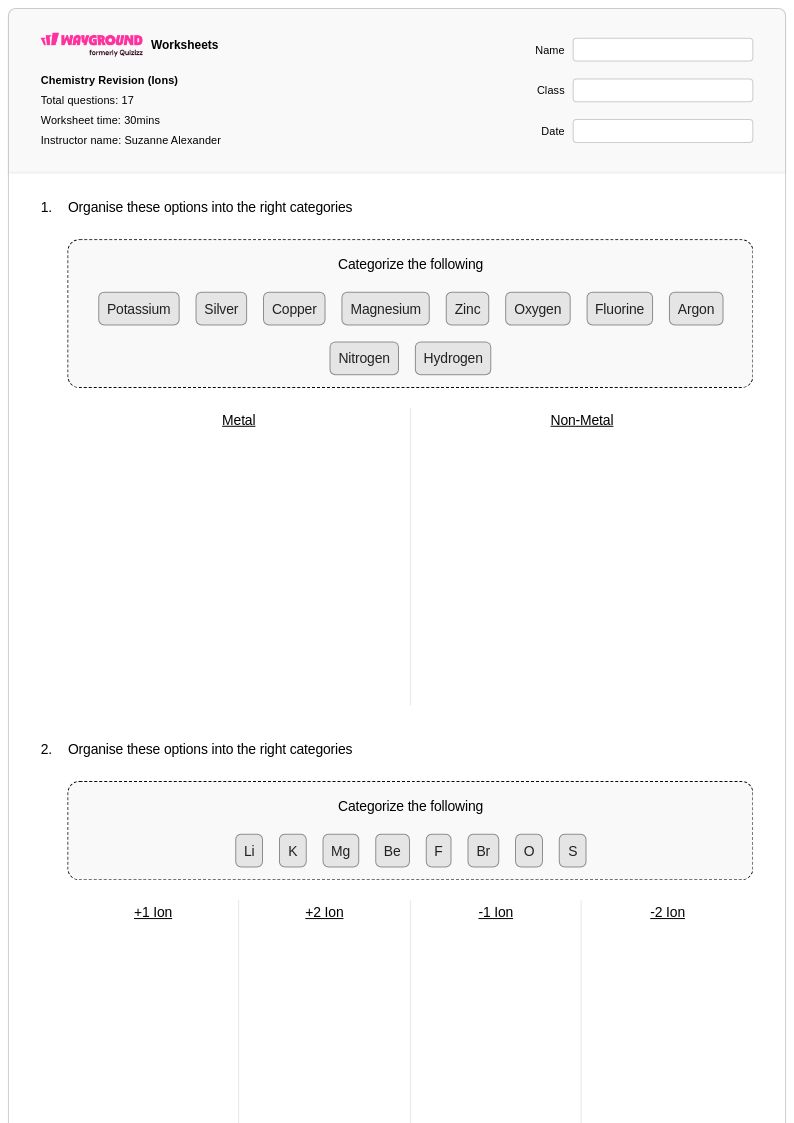
17 Q
9th - 12th
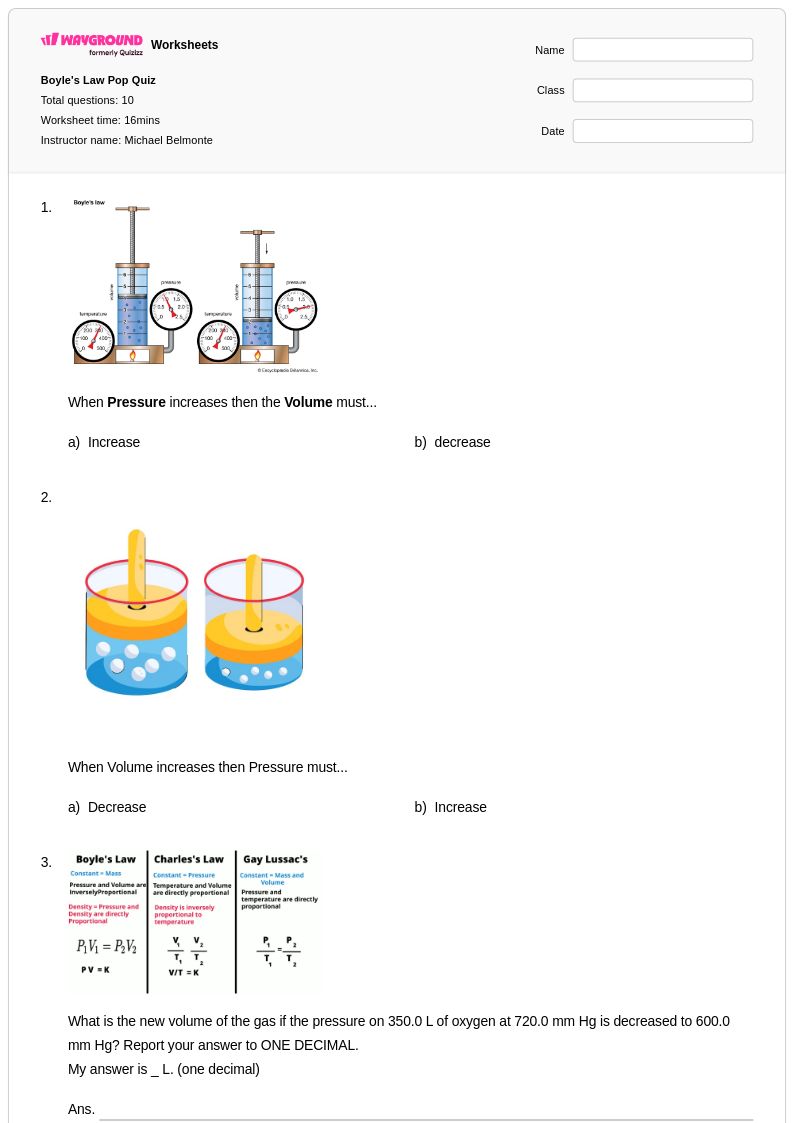
10 Q
9th - 12th
10 Q
11th

19 Q
9th - 11th
25 Q
10th - Uni

9 Q
10th - 11th
17 Q
10th - 11th

16 Q
2nd - Uni
19 Q
9th - 12th
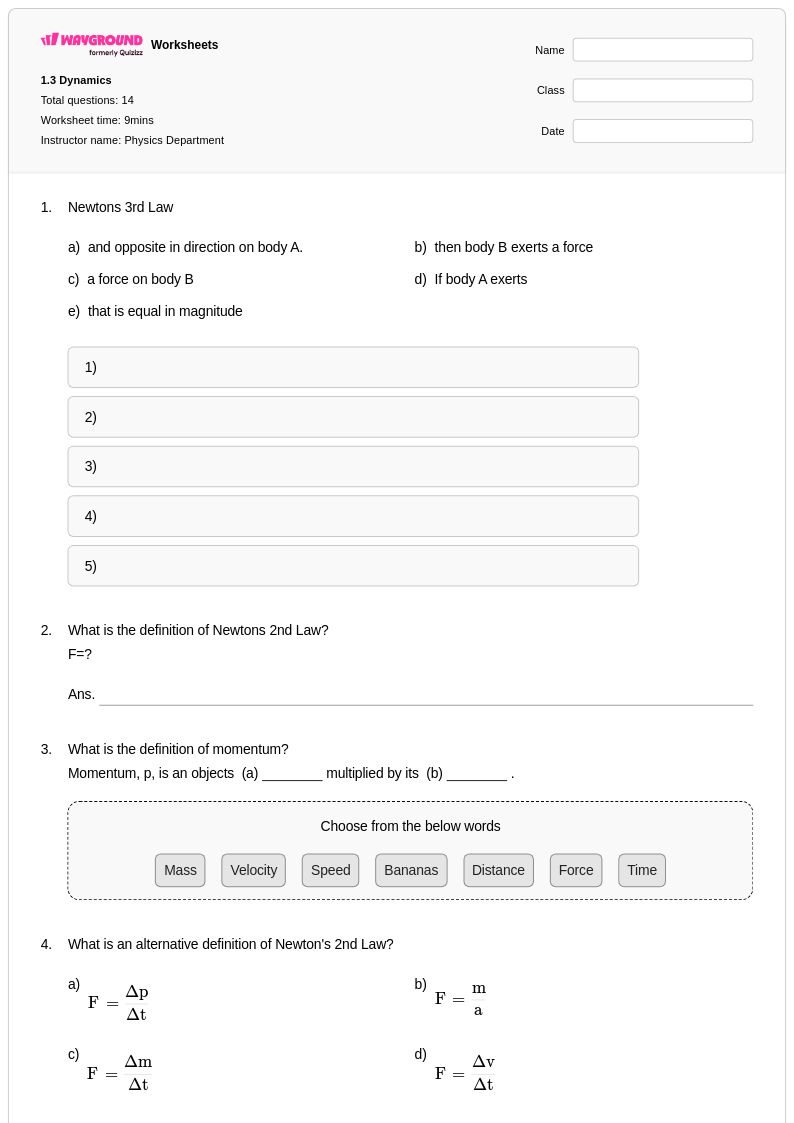
14 Q
11th
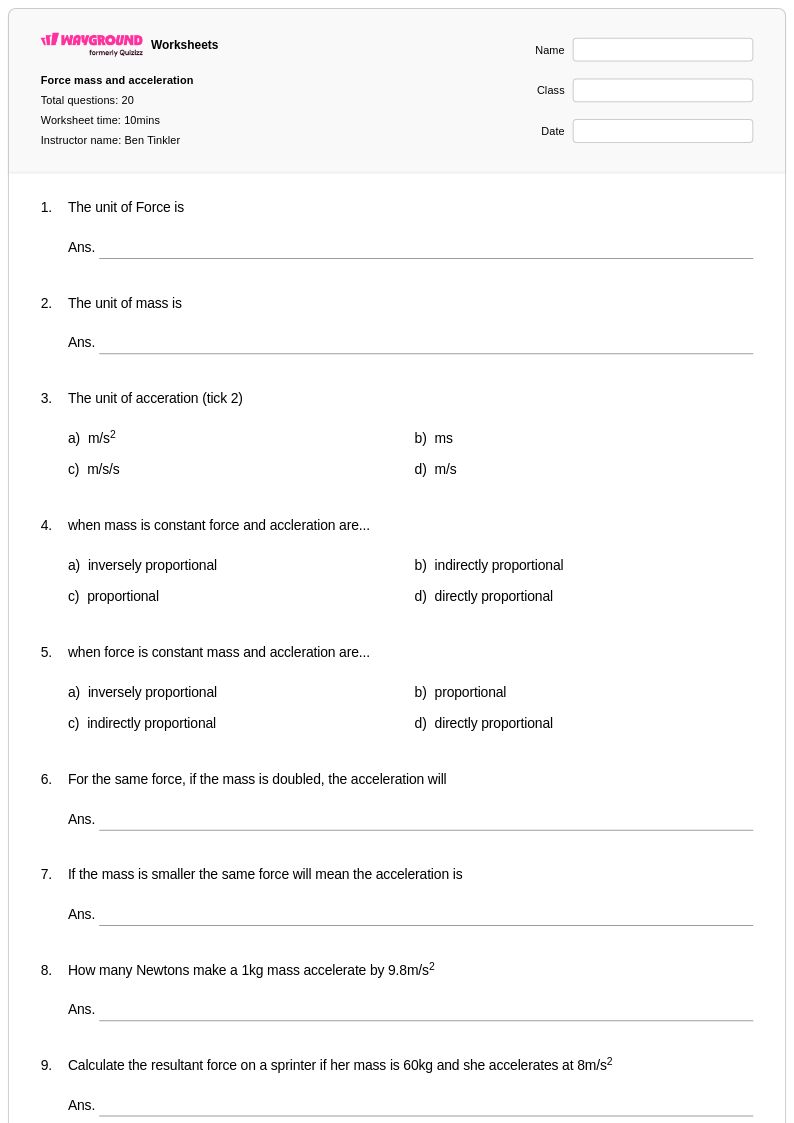
20 Q
9th - 11th

15 Q
9th - 12th

25 Q
11th

20 Q
8th - Uni

22 Q
11th

11 Q
11th

20 Q
11th

12 Q
11th
Explore Other Subject Worksheets for year 11
Explore printable Electrolysis and Faraday's Law worksheets for Year 11
Year 11 electrolysis and Faraday's Law worksheets available through Wayground provide comprehensive practice with electrochemical processes and quantitative calculations that form the foundation of advanced chemistry study. These expertly designed resources strengthen students' understanding of oxidation-reduction reactions at electrodes, the relationship between electric current and chemical change, and the mathematical principles governing electrolytic cells. Students work through practice problems that cover calculating the amount of substance produced or consumed during electrolysis, determining current efficiency, and applying Faraday's constants to real-world scenarios. Each worksheet includes detailed answer keys that guide learners through step-by-step solutions, while free printable pdf formats ensure accessibility for both classroom instruction and independent study sessions.
Wayground's extensive collection of teacher-created electrolysis and Faraday's Law resources draws from millions of educational materials specifically curated for Year 11 chemistry instruction. The platform's advanced search and filtering capabilities allow educators to quickly locate worksheets aligned with curriculum standards and differentiate instruction based on individual student needs. Teachers can customize existing materials or create entirely new practice sets, with flexible options for both digital delivery and printable pdf distribution. These comprehensive tools support lesson planning by providing ready-to-use assessments and practice materials, while also serving as valuable resources for targeted remediation when students struggle with electrochemical calculations and enrichment opportunities for advanced learners seeking additional challenge in quantitative chemistry applications.
