
15 Q
10th - Uni

20 Q
11th

15 Q
11th - Uni
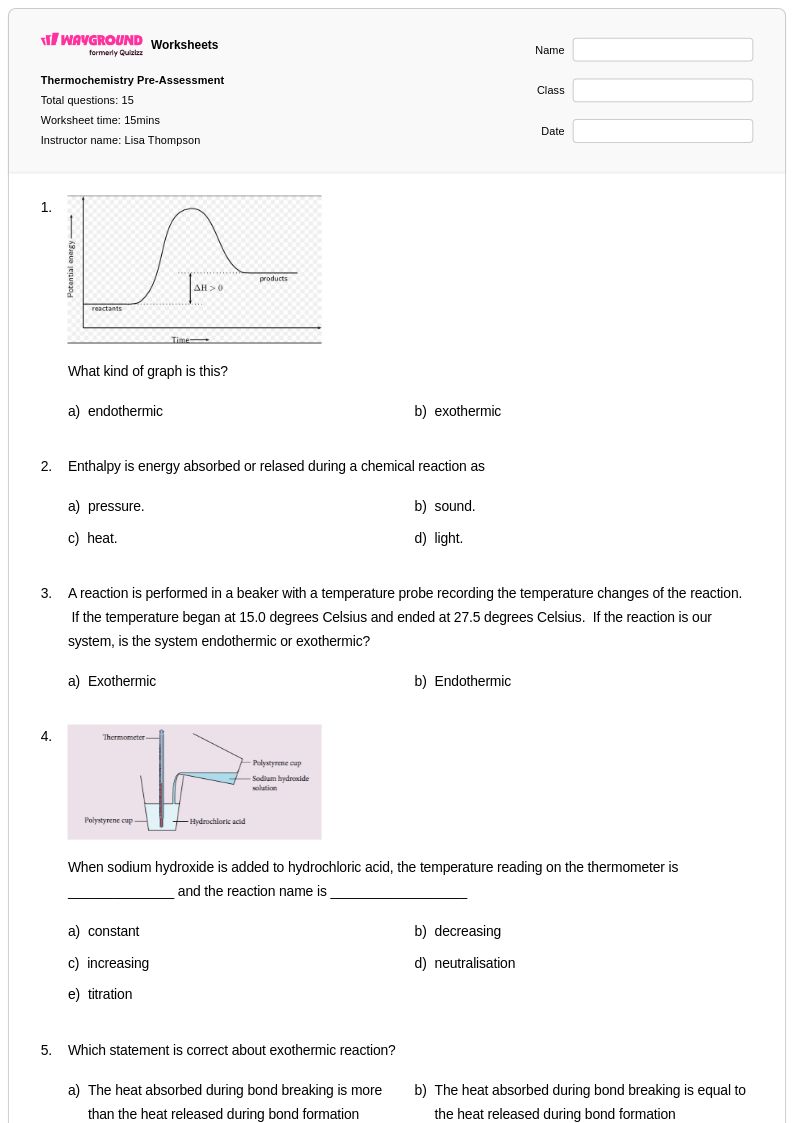
15 Q
10th - Uni

15 Q
10th - Uni

16 Q
9th - 12th
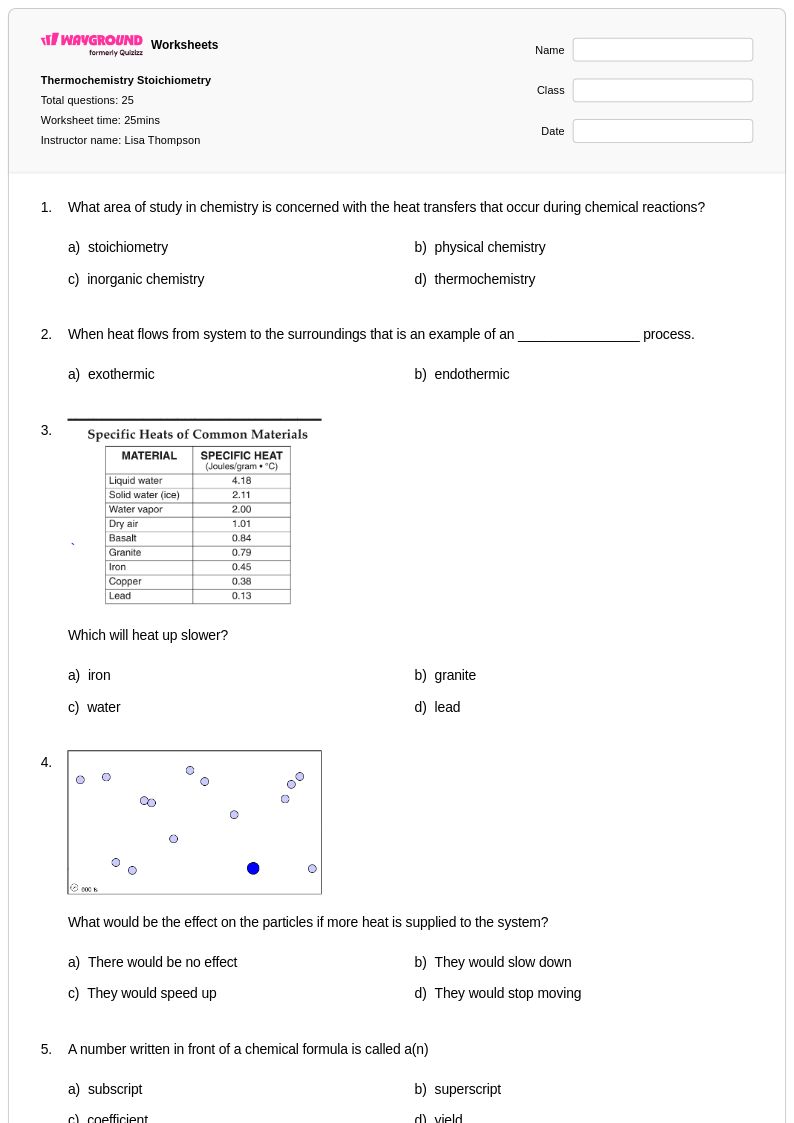
25 Q
10th - Uni

15 Q
10th - Uni
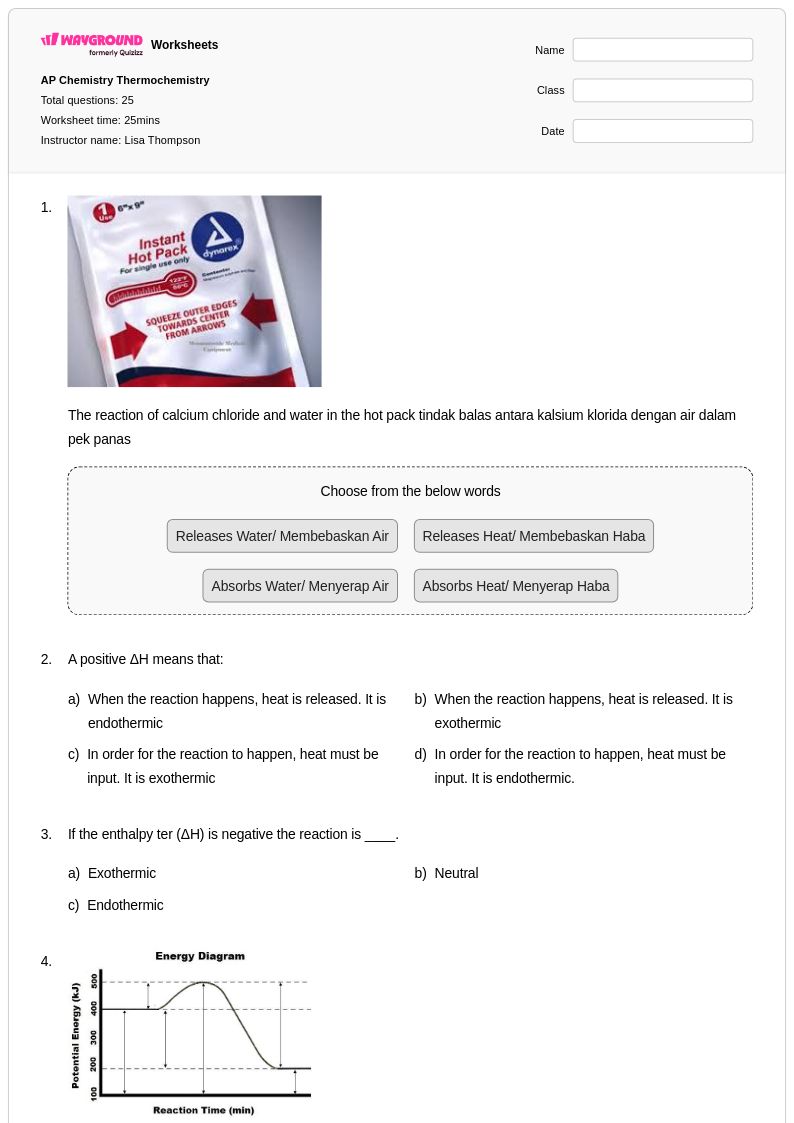
25 Q
10th - Uni
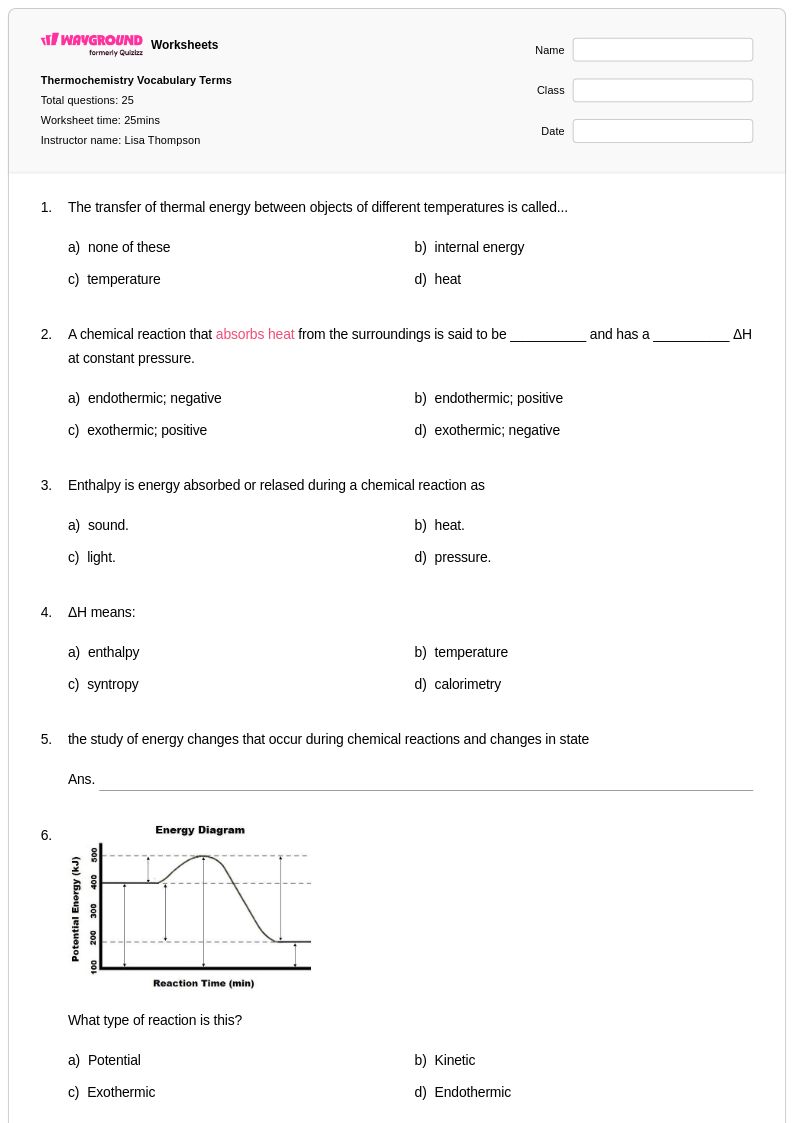
25 Q
10th - Uni
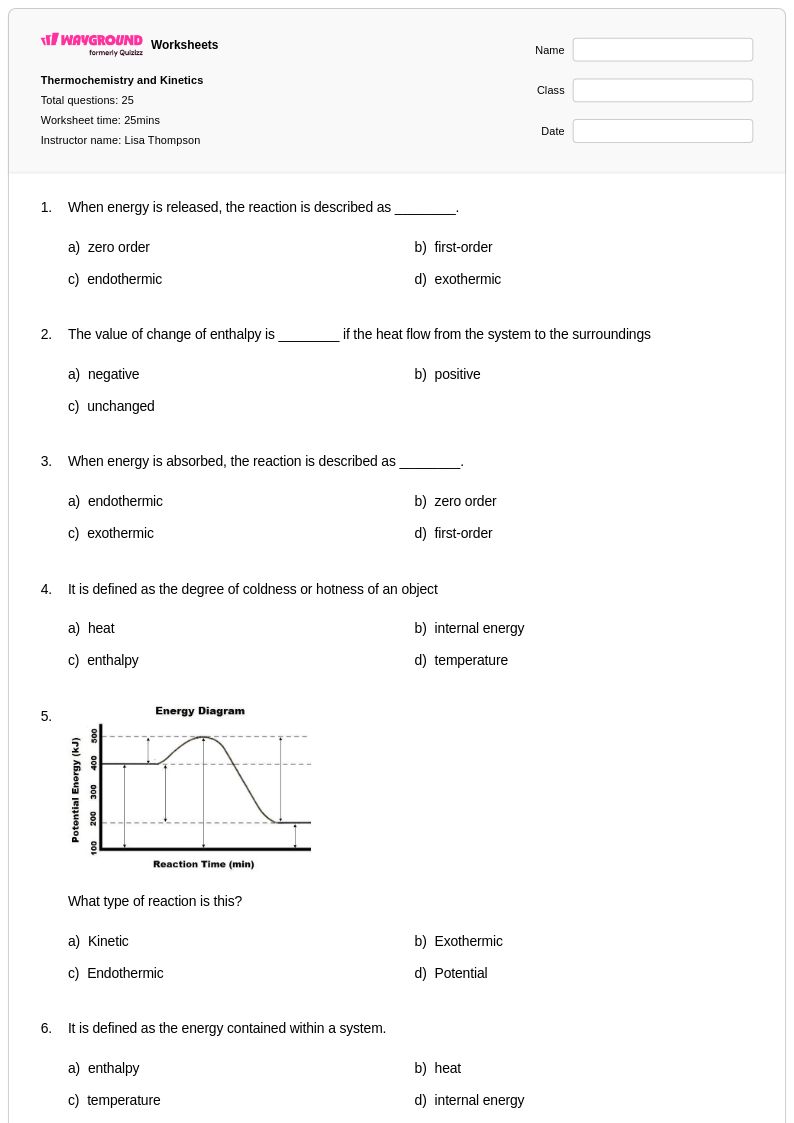
25 Q
10th - Uni

25 Q
10th - Uni

16 Q
9th - 12th

15 Q
10th - Uni

15 Q
10th - Uni
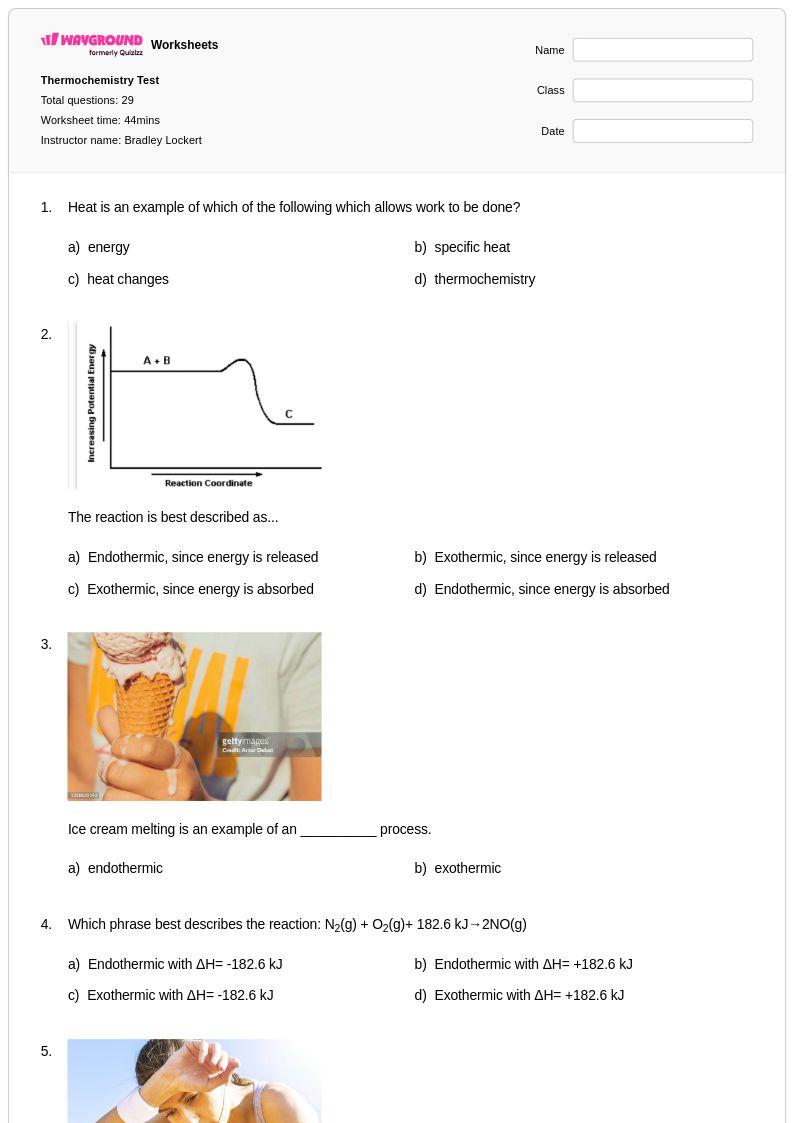
29 Q
9th - 12th

76 Q
10th - 12th

15 Q
10th - Uni
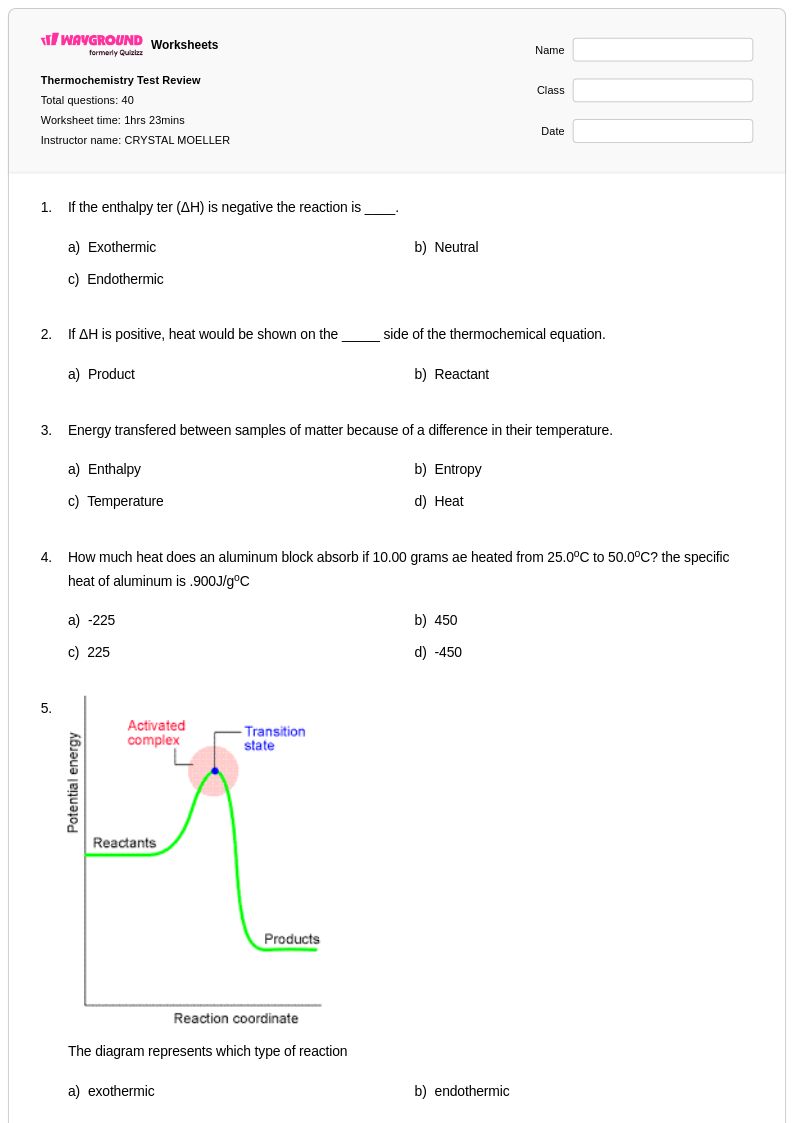
40 Q
10th - 12th

25 Q
11th - Uni

15 Q
10th - 12th
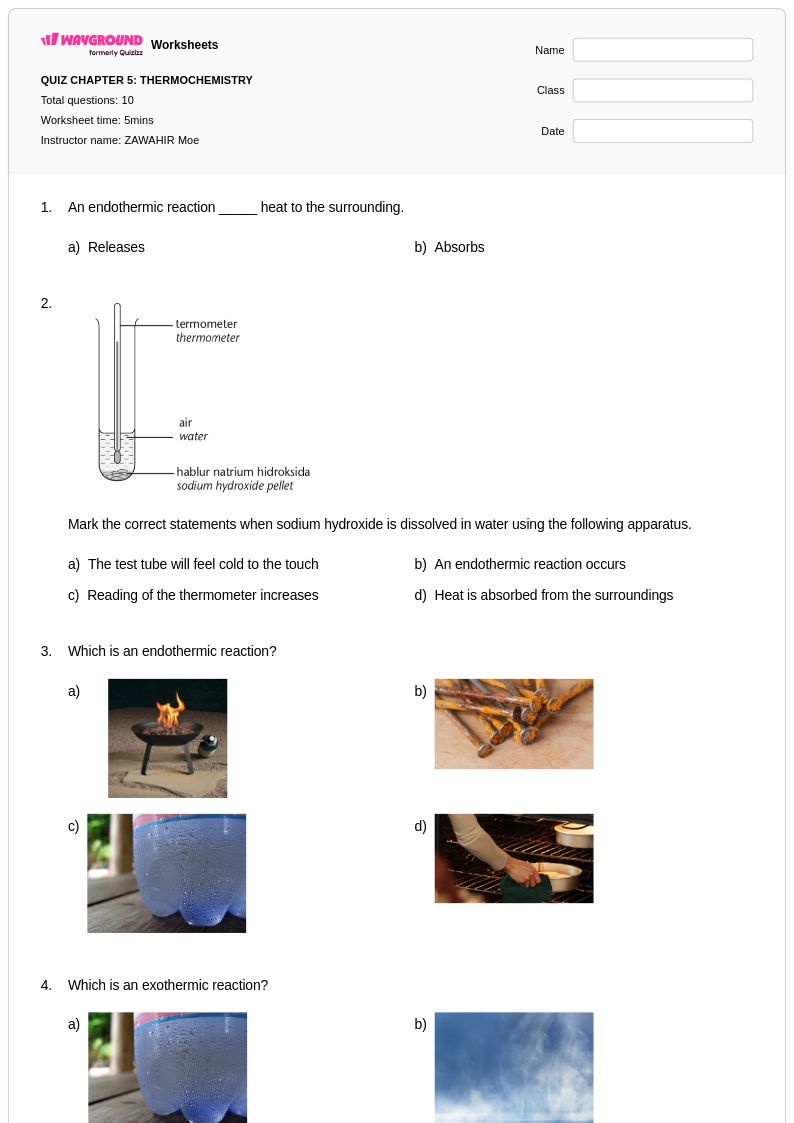
10 Q
10th - 12th
25 Q
11th - Uni

25 Q
10th - Uni
Explore Other Subject Worksheets for year 11
Explore printable Thermochemistry worksheets for Year 11
Thermochemistry worksheets for Year 11 students available through Wayground (formerly Quizizz) provide comprehensive coverage of energy changes in chemical reactions and the quantitative relationships between heat and chemical processes. These expertly designed resources strengthen critical skills including calculating enthalpy changes, applying Hess's law, interpreting calorimetry data, and understanding the relationship between bond energies and reaction thermodynamics. Students develop proficiency in solving complex practice problems involving heat of formation, heat of combustion, and spontaneity predictions using thermodynamic principles. Each worksheet collection includes detailed answer keys and is available as free printable pdf resources, enabling students to master the mathematical and conceptual foundations essential for advanced chemistry coursework.
Wayground (formerly Quizizz) supports chemistry educators with millions of teacher-created thermochemistry resources that streamline lesson planning and enhance student learning outcomes. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets aligned with specific curriculum standards and learning objectives, while differentiation tools enable customization for varying student ability levels within Year 11 classrooms. These thermochemistry materials are available in both printable and digital formats, including downloadable pdf versions, providing maximum flexibility for classroom instruction, homework assignments, and laboratory supplement activities. Teachers can efficiently implement these resources for targeted skill practice, remediation of challenging concepts like entropy and Gibbs free energy, and enrichment opportunities that prepare students for advanced placement chemistry and post-secondary science programs.
