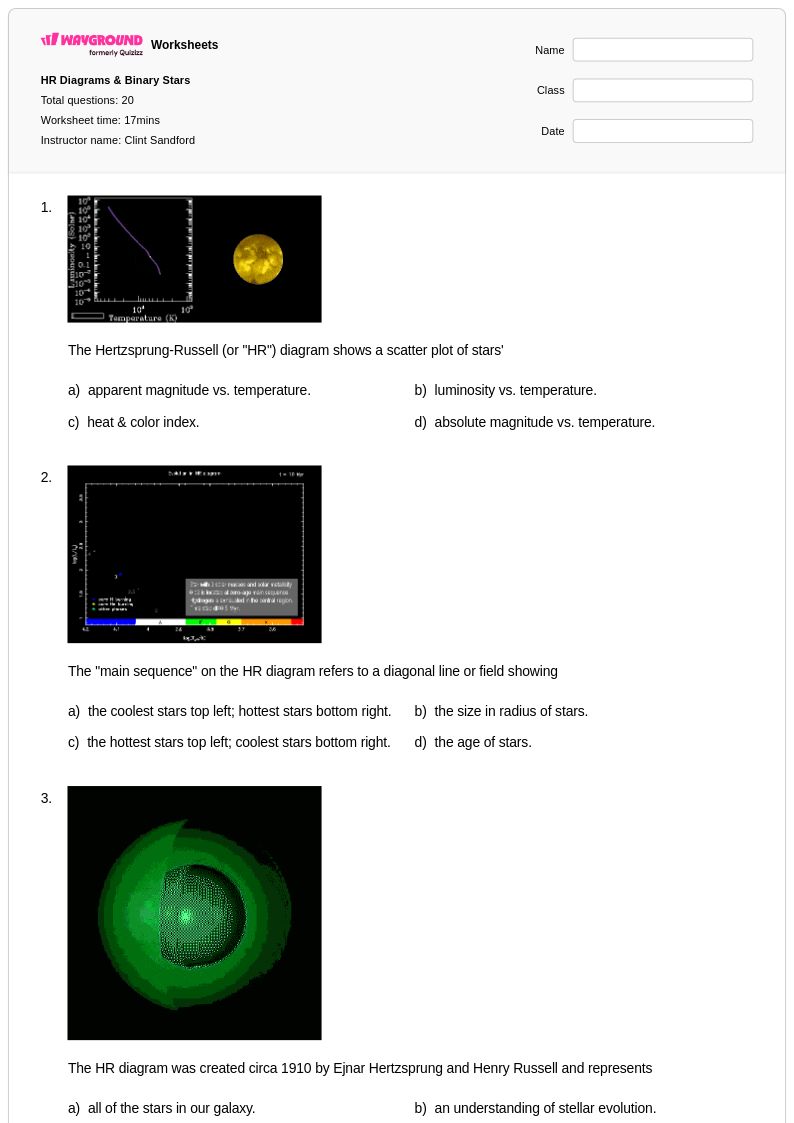
20 Q
9th - 12th
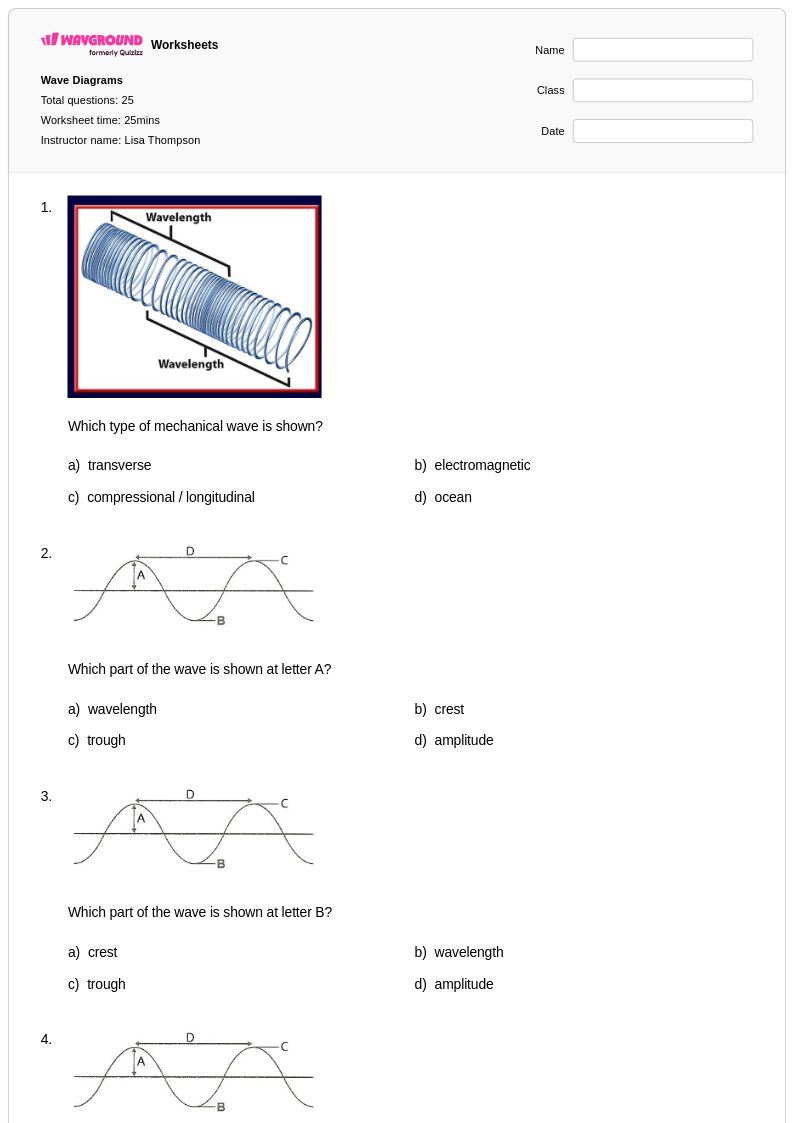
25 Q
8th - Uni

18 Q
11th
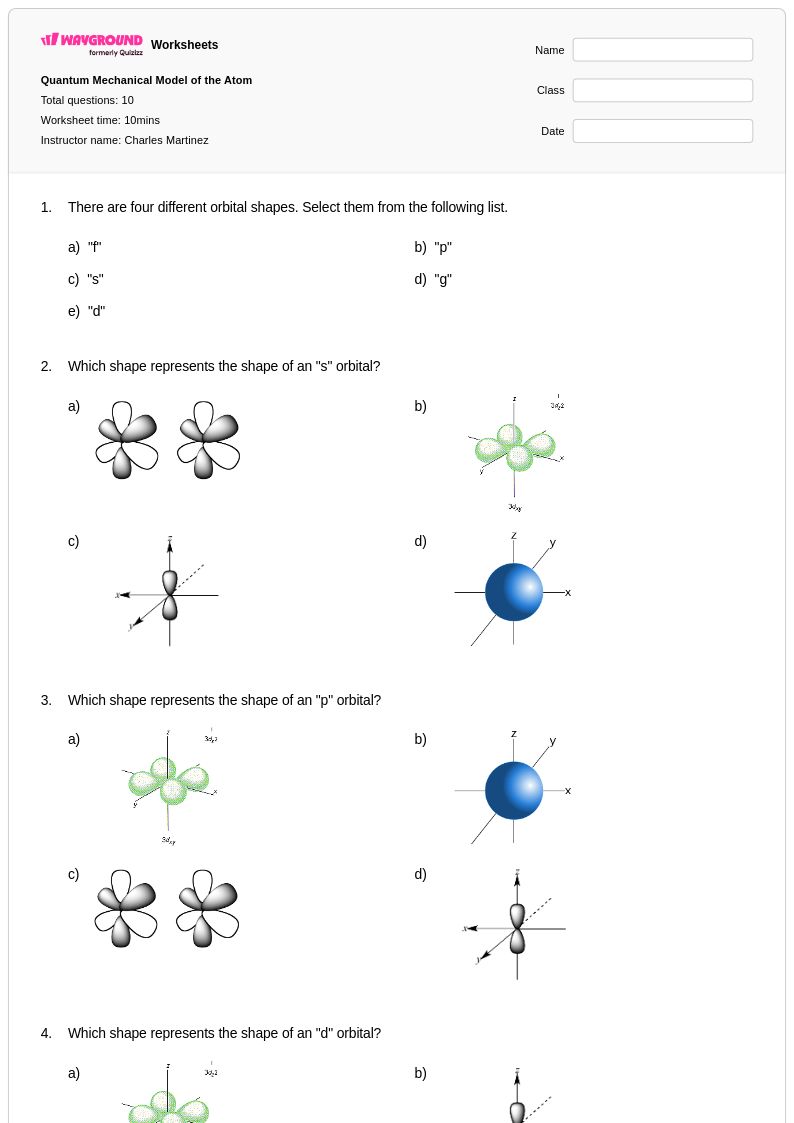
10 Q
9th - 12th
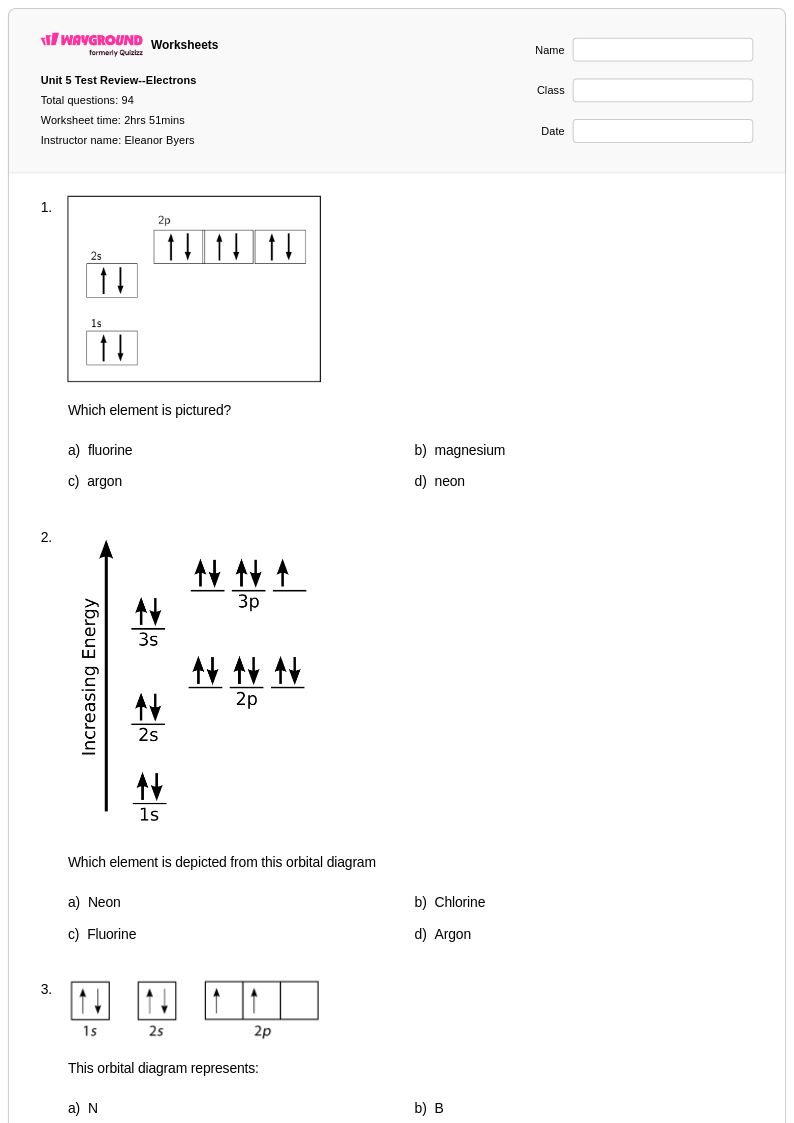
94 Q
9th - 12th
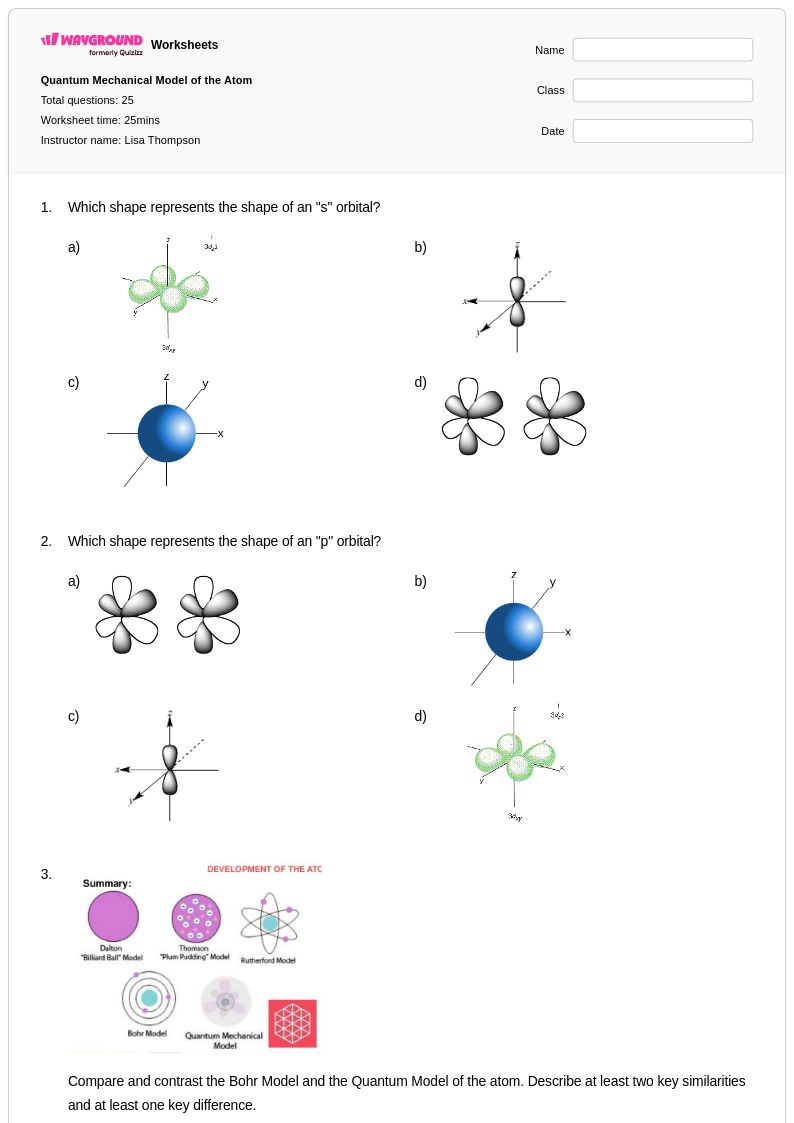
25 Q
9th - Uni
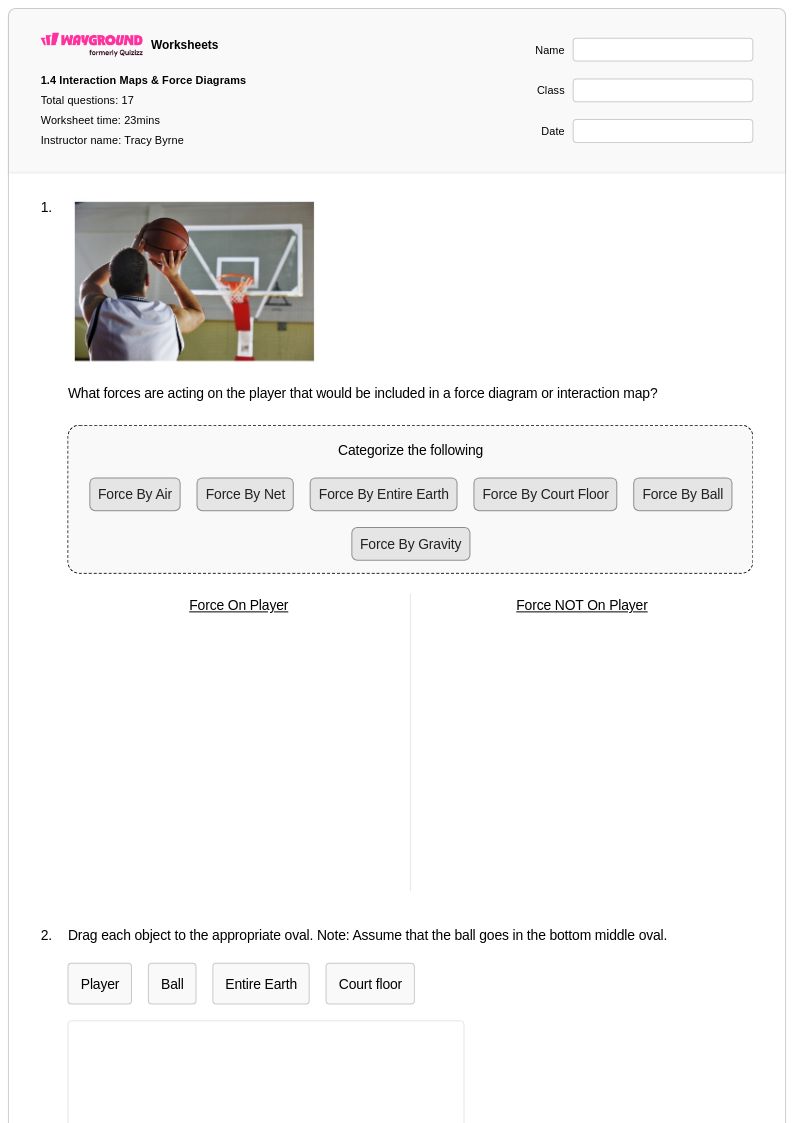
17 Q
9th - 12th
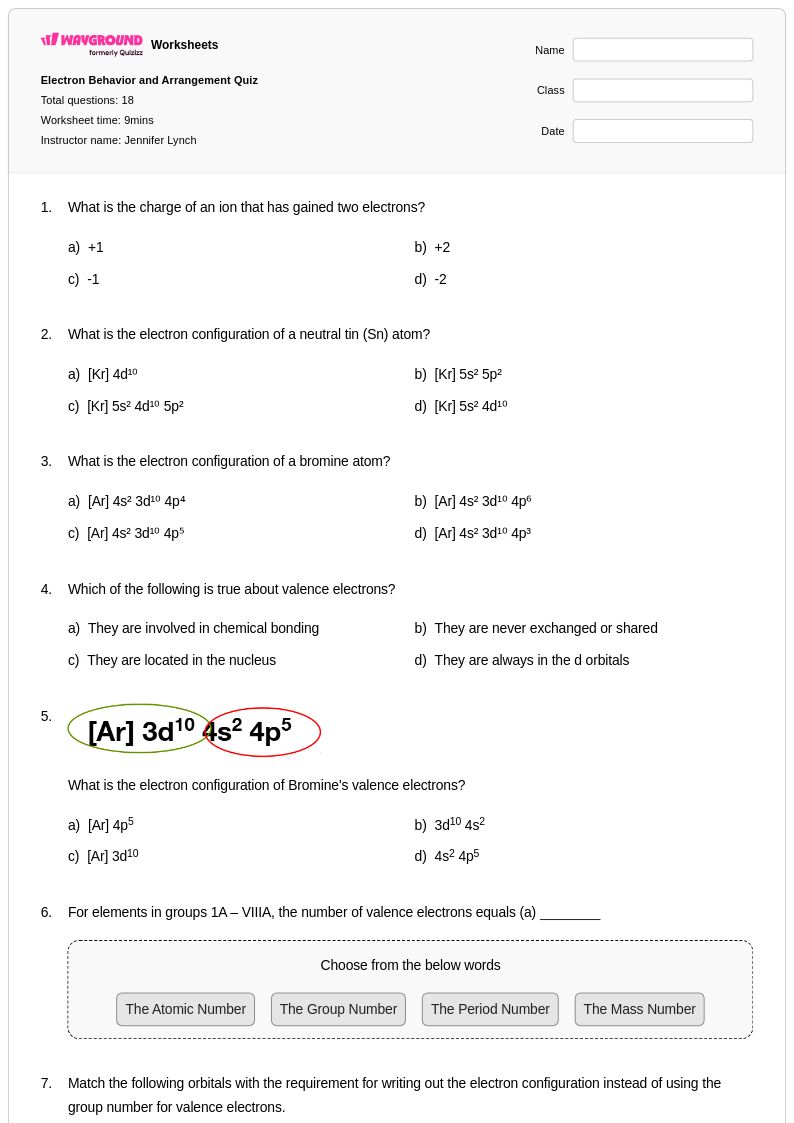
18 Q
11th - Uni

222 Q
11th
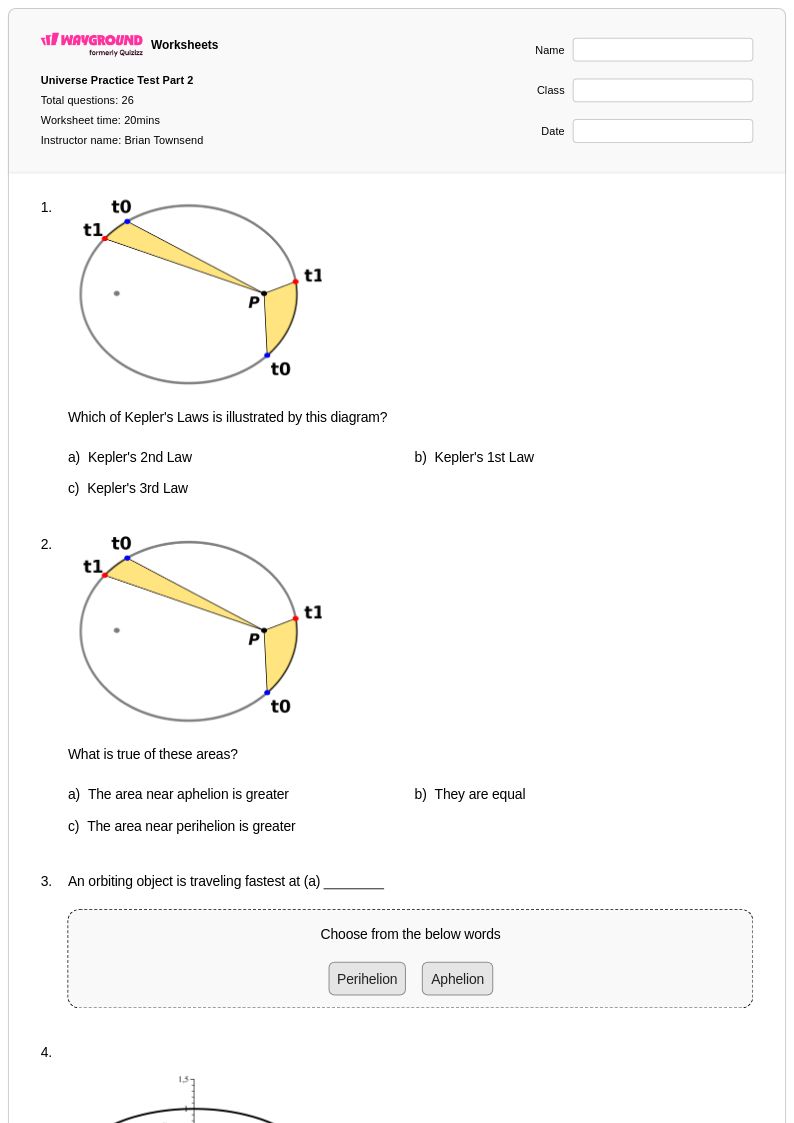
26 Q
11th

6 Q
11th
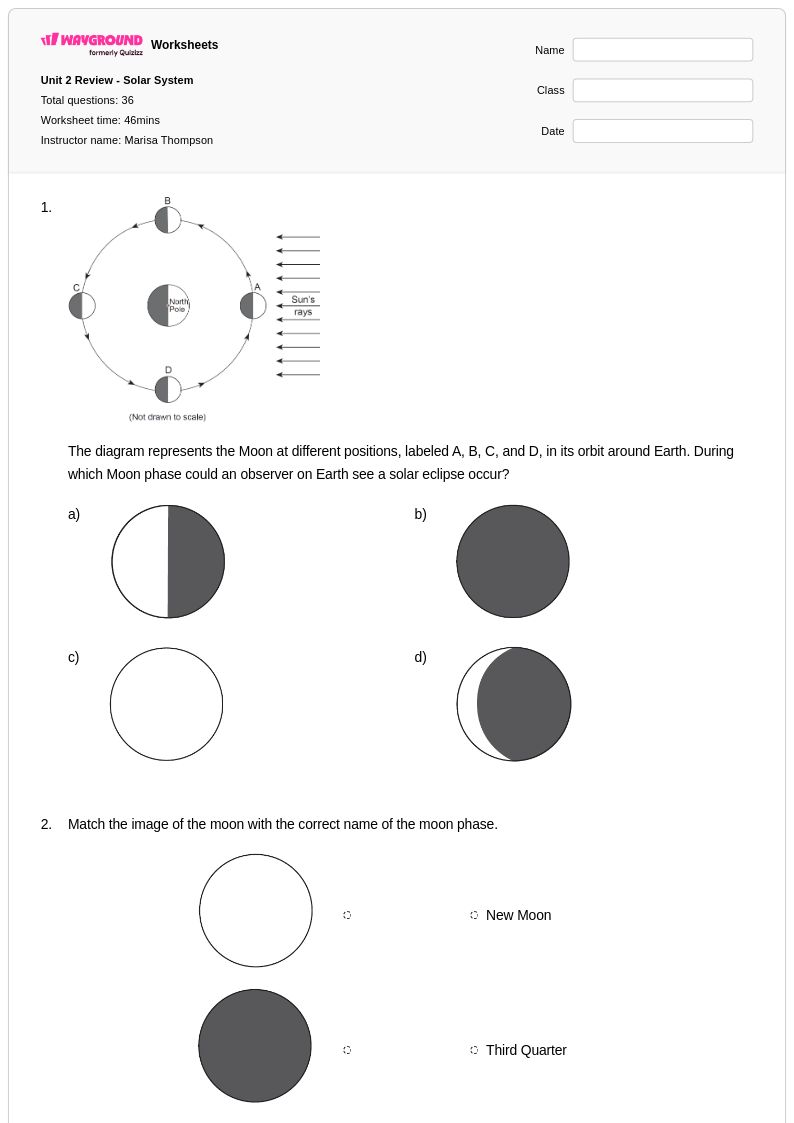
36 Q
9th - 12th
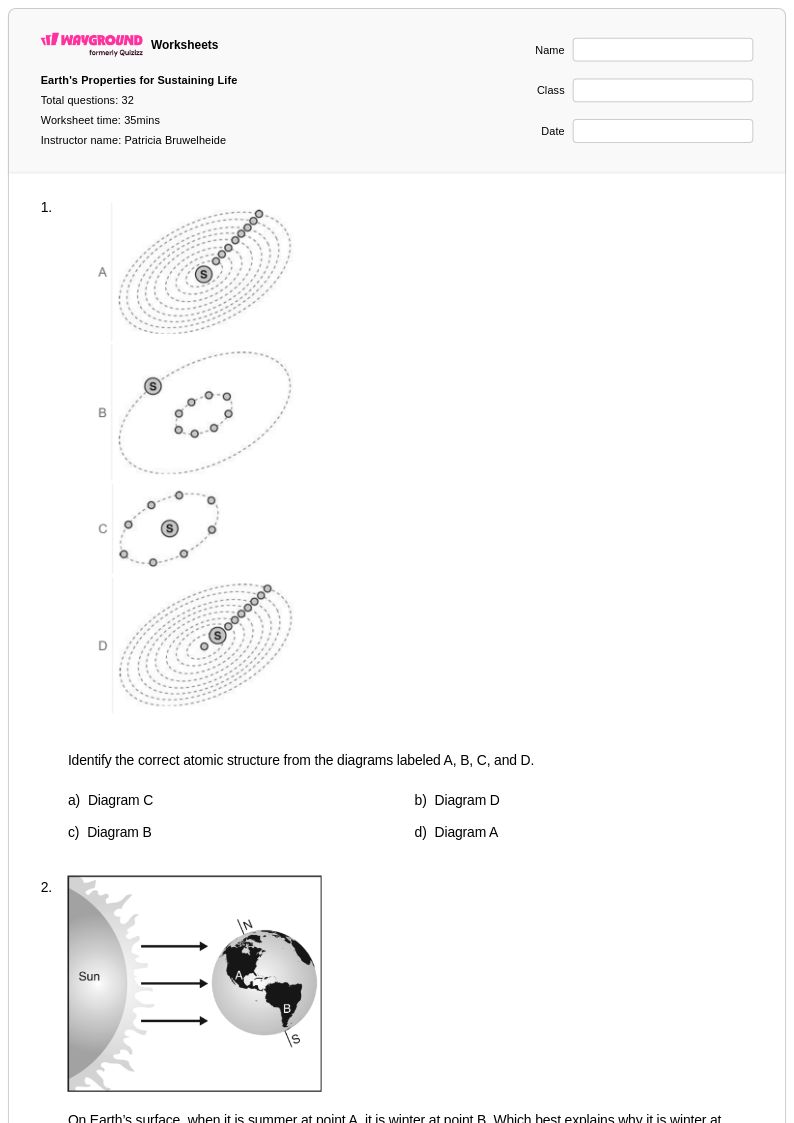
32 Q
6th - Uni

50 Q
9th - 12th
30 Q
9th - 12th

20 Q
9th - 12th

6 Q
11th

10 Q
9th - 12th

20 Q
11th
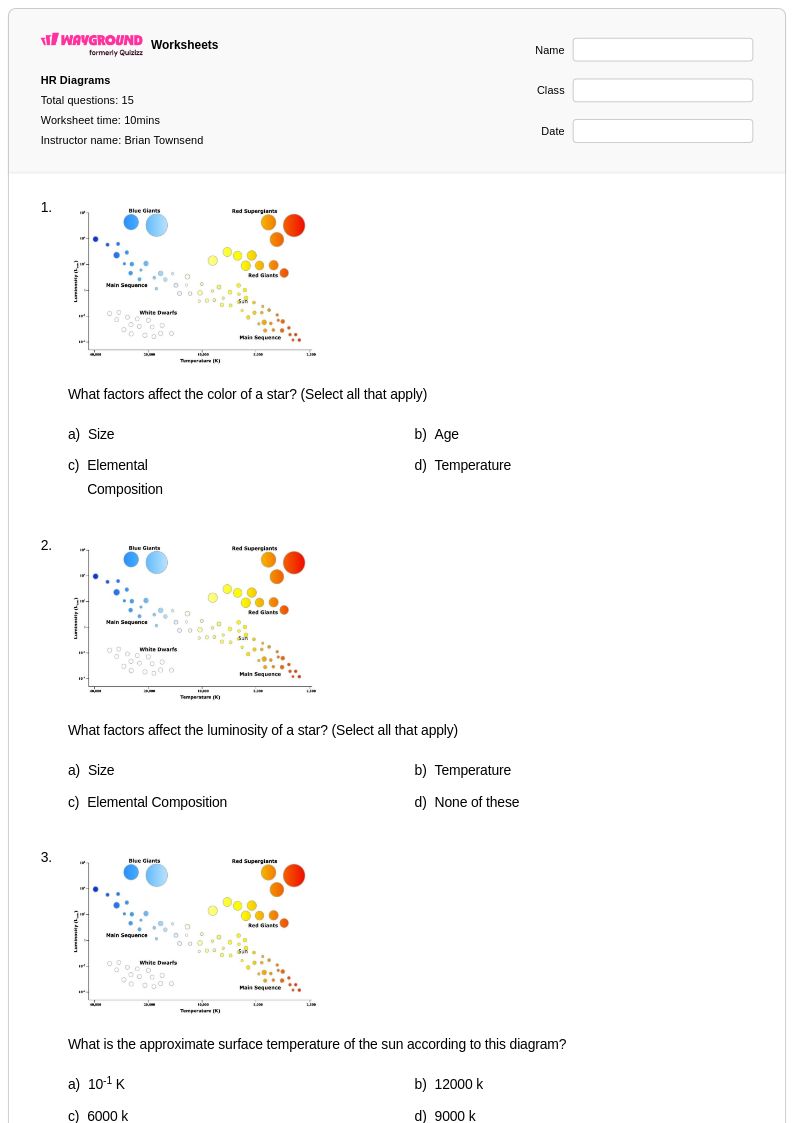
15 Q
11th
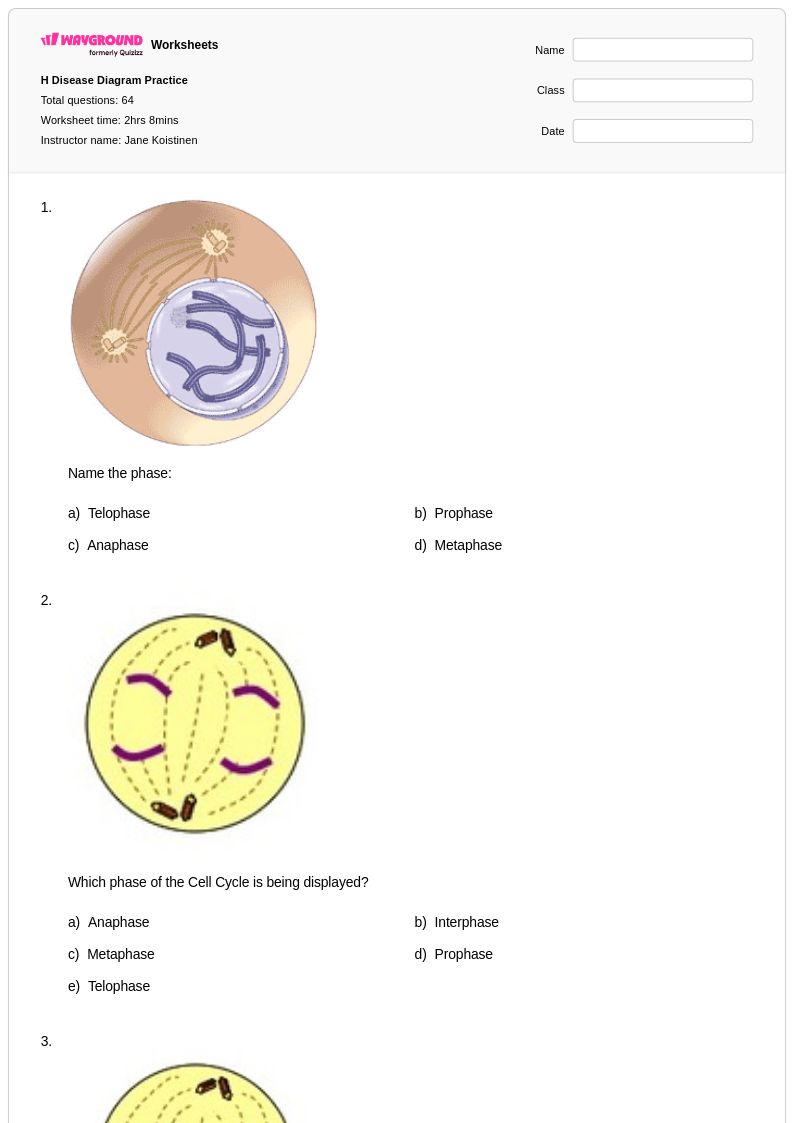
64 Q
9th - 12th
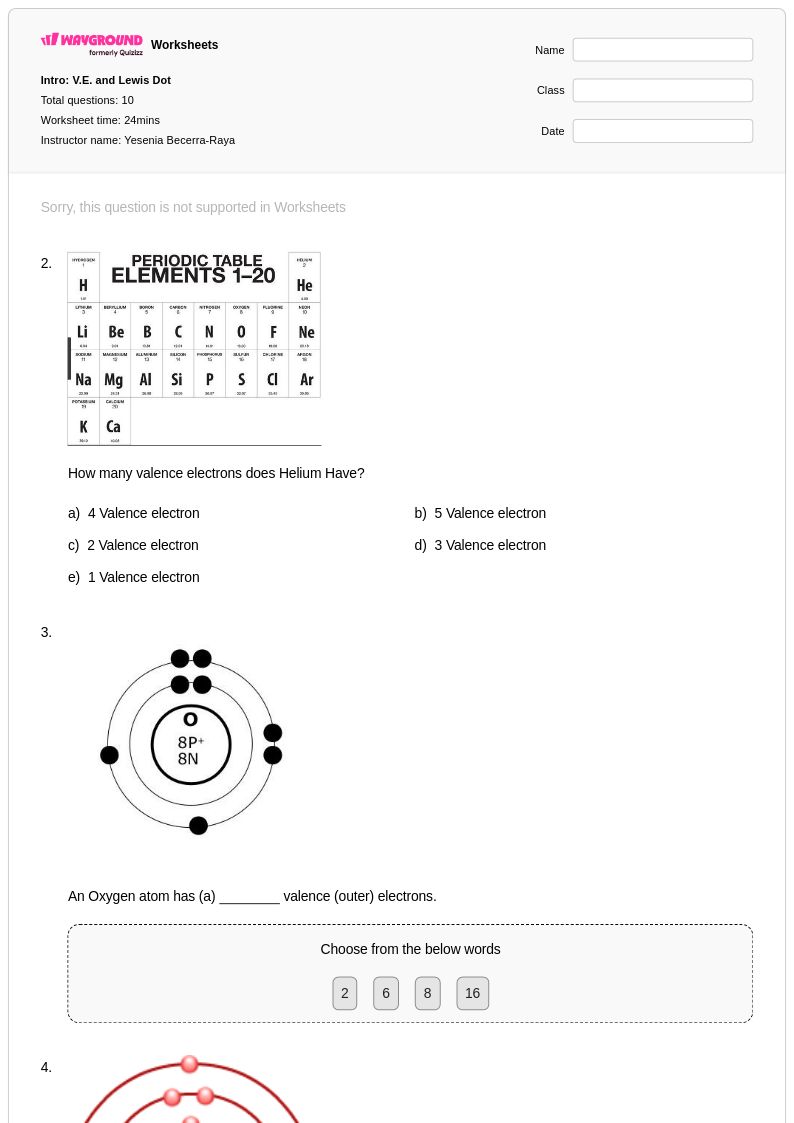
25 Q
9th - 12th
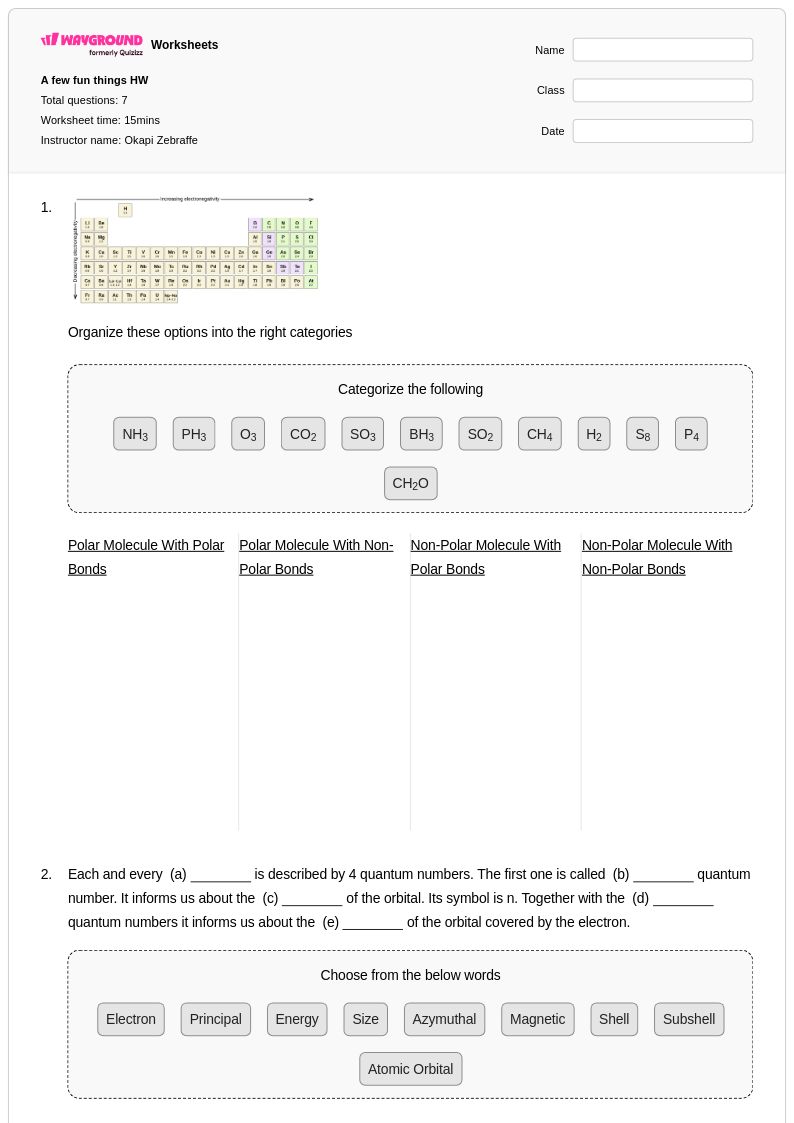
7 Q
11th
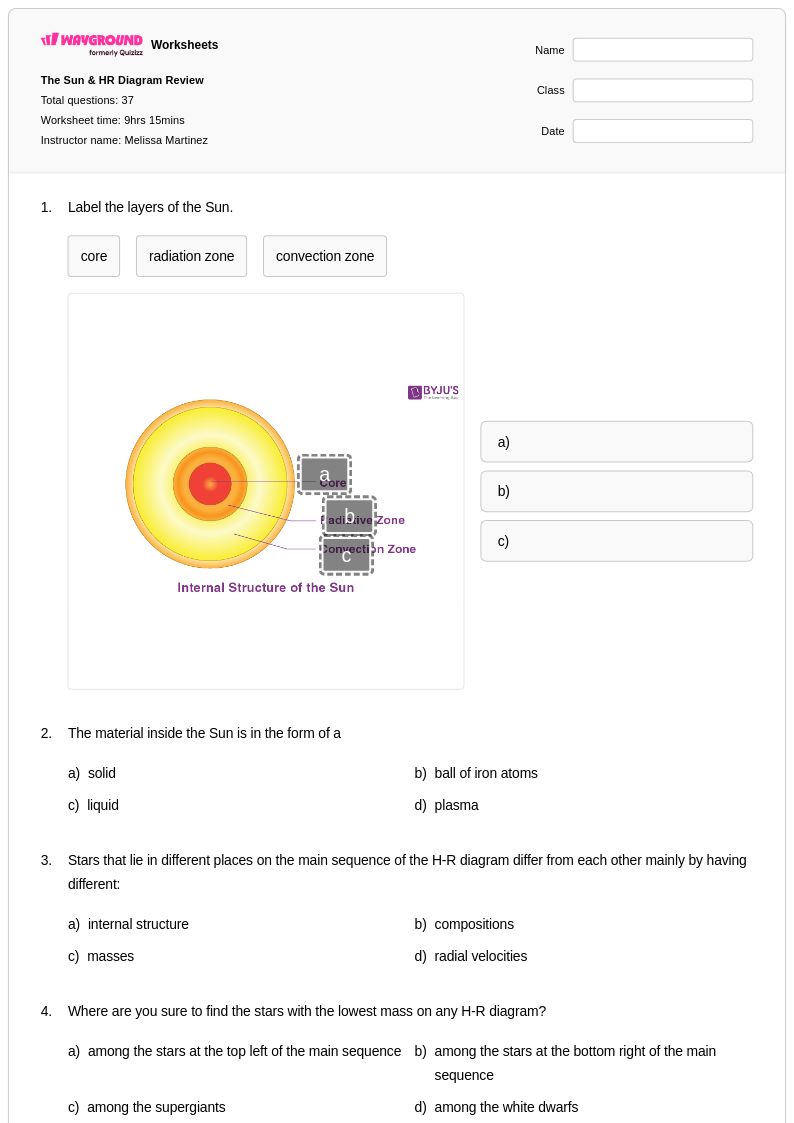
37 Q
9th - 12th
Explore Other Subject Worksheets for year 11
Explore printable Orbital Diagrams worksheets for Year 11
Orbital diagrams represent a fundamental concept in Year 11 chemistry that helps students visualize electron arrangements within atoms and understand the relationship between electron configuration and chemical behavior. Wayground's comprehensive collection of orbital diagram worksheets provides students with structured practice problems that develop their ability to construct accurate orbital diagrams using Hund's rule, the Aufbau principle, and Pauli exclusion principle. These free printables include detailed answer keys that allow students to self-check their work and identify common misconceptions about electron spin pairing and orbital filling order. The worksheets progress systematically from simple s and p orbital diagrams to more complex d and f orbital arrangements, strengthening students' foundational understanding of atomic structure and preparing them for advanced topics in chemical bonding and molecular geometry.
Wayground supports chemistry educators with millions of teacher-created orbital diagram resources that can be easily searched, filtered, and customized to meet diverse classroom needs. The platform's robust collection includes both printable pdf worksheets and interactive digital formats, allowing teachers to differentiate instruction based on student readiness levels and learning preferences. Standards-aligned content ensures that orbital diagram practice aligns with curriculum expectations, while flexible customization tools enable educators to modify existing worksheets or create new ones tailored to specific learning objectives. These resources prove invaluable for lesson planning, targeted remediation of electron configuration concepts, enrichment activities for advanced learners, and regular skill practice that reinforces the spatial reasoning and systematic thinking required to master orbital theory in chemistry.
