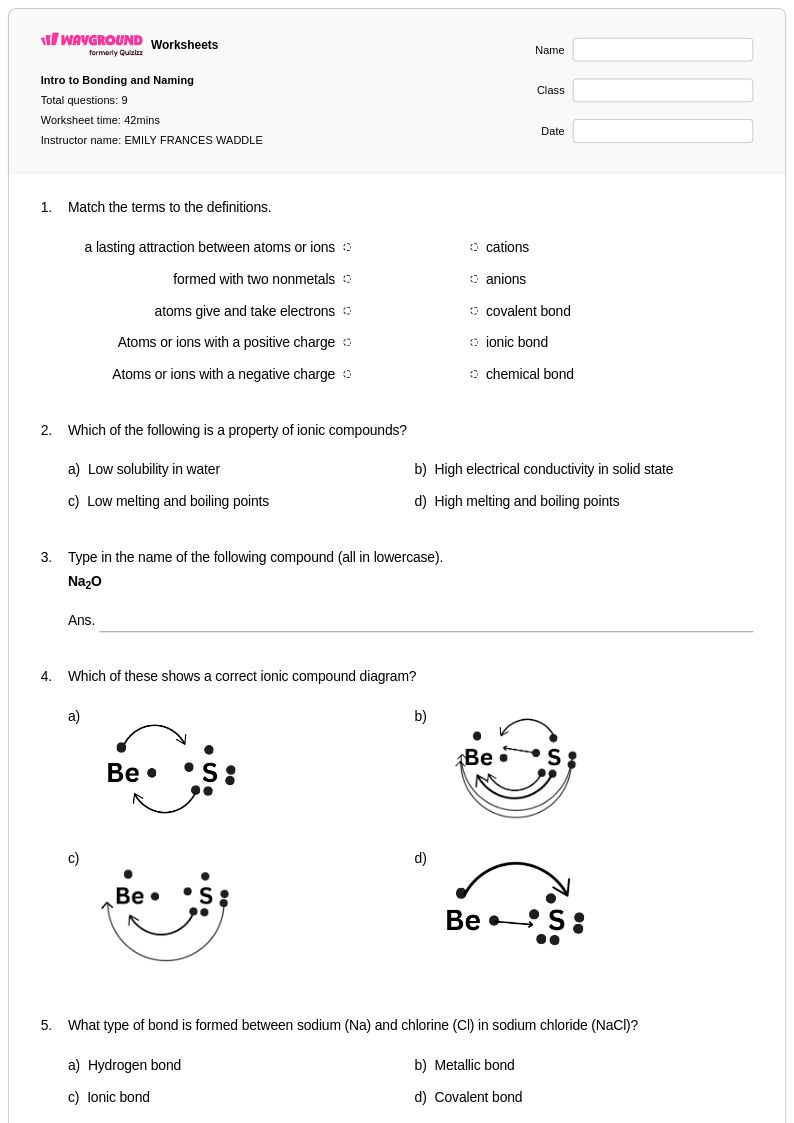
9 Q
9th - 12th

58 Q
9th - 12th

16 Q
9th - 12th
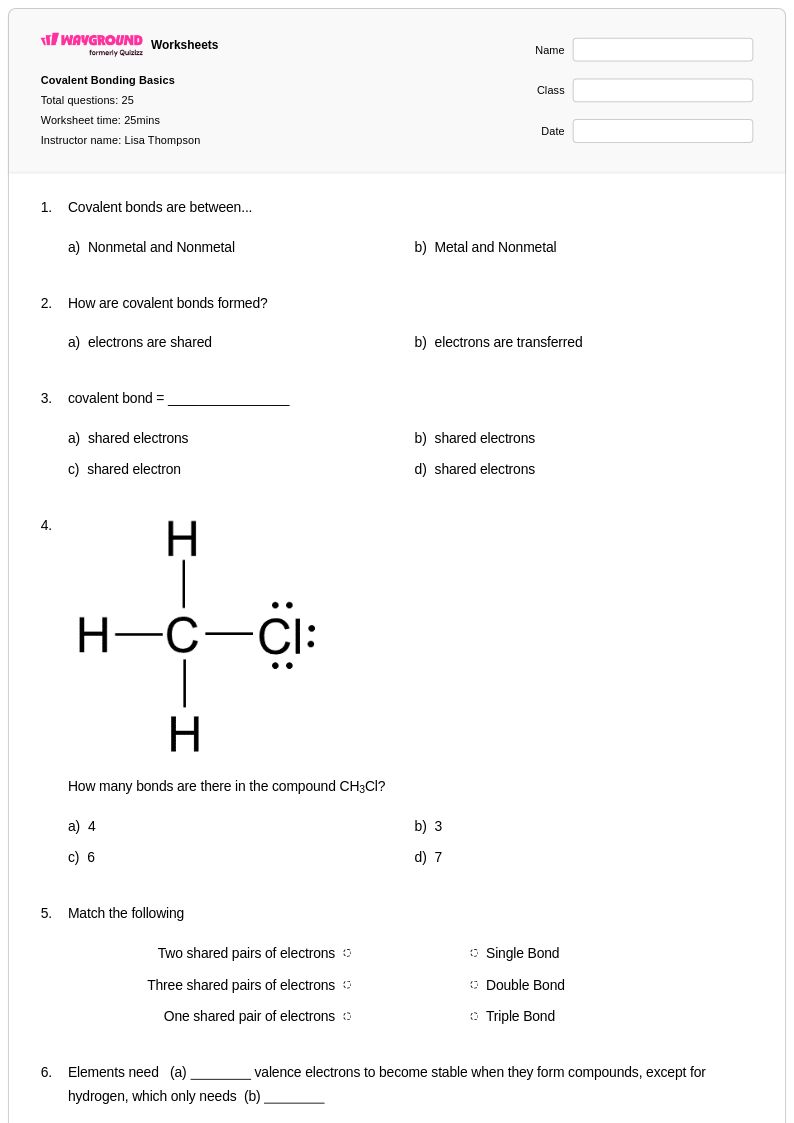
25 Q
9th - Uni
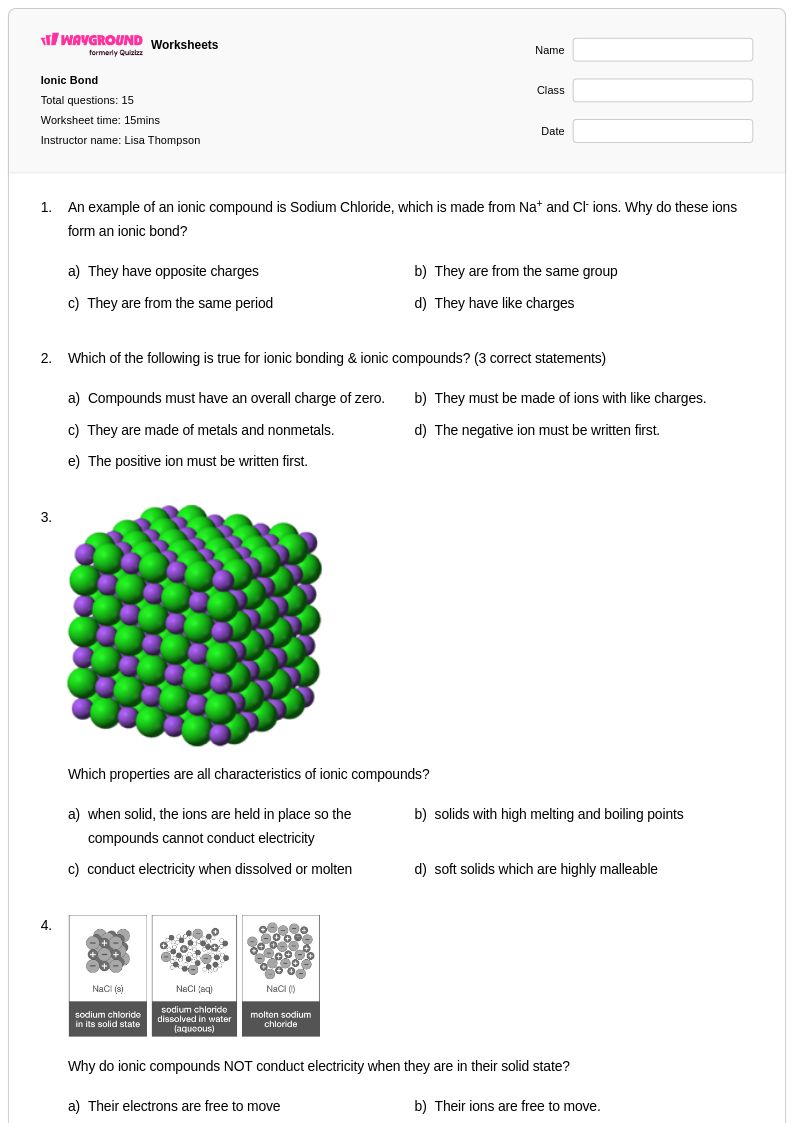
15 Q
10th - Uni

15 Q
10th - Uni
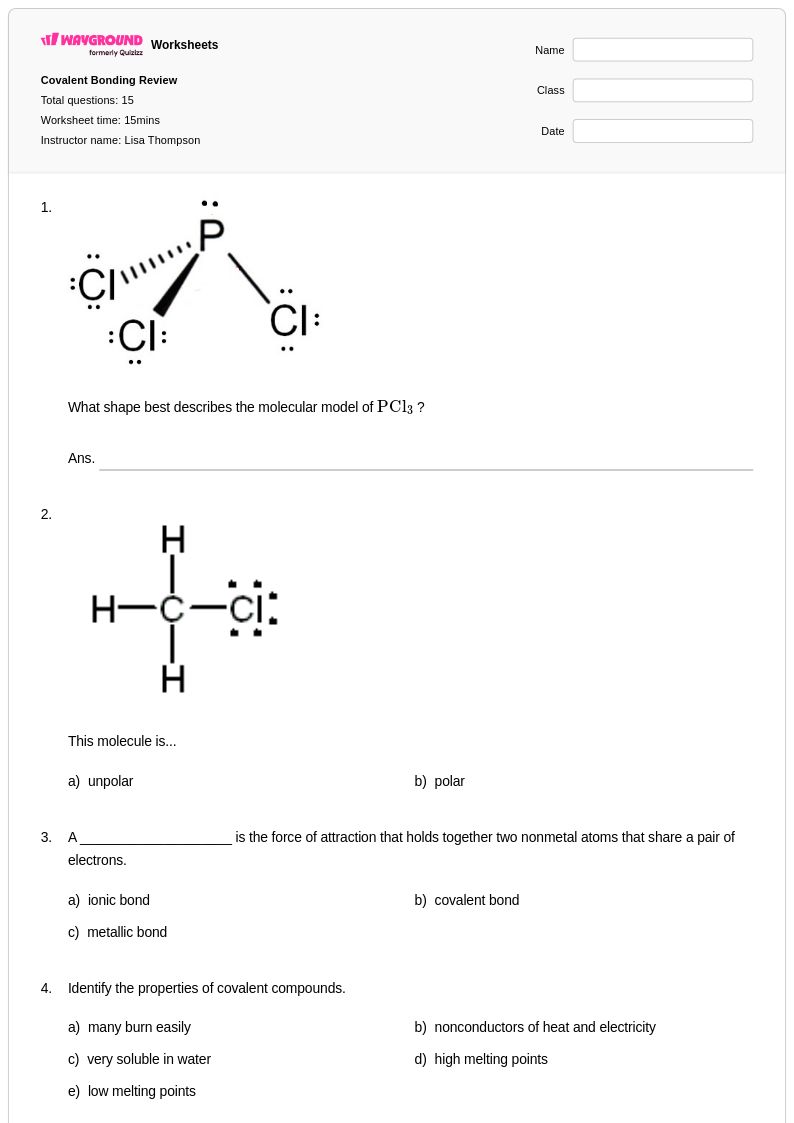
15 Q
10th - Uni

25 Q
9th - Uni

15 Q
11th
15 Q
11th

26 Q
11th - Uni

25 Q
10th - Uni

32 Q
9th - 12th
15 Q
8th - Uni

20 Q
9th - 12th

12 Q
11th

30 Q
11th

42 Q
11th
10 Q
11th

67 Q
11th
20 Q
9th - 12th

68 Q
9th - 12th

21 Q
9th - 12th

18 Q
9th - 12th
Explore Ionic and Covalent Compounds Worksheets by Grades
Explore Other Subject Worksheets for year 11
Explore printable Ionic and Covalent Compounds worksheets for Year 11
Ionic and covalent compounds represent fundamental concepts that Year 11 chemistry students must master to understand chemical bonding and molecular behavior. Wayground's extensive worksheet collection provides comprehensive practice problems that guide students through identifying compound types, predicting chemical formulas, and analyzing bonding patterns between metals and nonmetals. These carefully designed printables strengthen critical thinking skills by challenging students to differentiate between ionic compounds formed through electron transfer and covalent compounds created by electron sharing. Each worksheet comes with detailed answer keys that support independent learning and self-assessment, while the free pdf format ensures accessibility for both classroom instruction and home study sessions.
Wayground's platform empowers educators with millions of teacher-created resources specifically tailored for ionic and covalent compound instruction, featuring robust search and filtering capabilities that align with chemistry curriculum standards. Teachers can easily customize worksheets to match their students' diverse learning needs, whether providing foundational practice for struggling learners or advanced problem-solving challenges for accelerated students. The flexible digital and printable formats facilitate seamless lesson planning, allowing educators to incorporate these resources into remediation sessions, enrichment activities, or regular skill practice routines. This comprehensive approach ensures that students develop a solid foundation in chemical bonding principles while teachers maintain the flexibility to adapt instruction based on individual classroom requirements and pacing needs.
