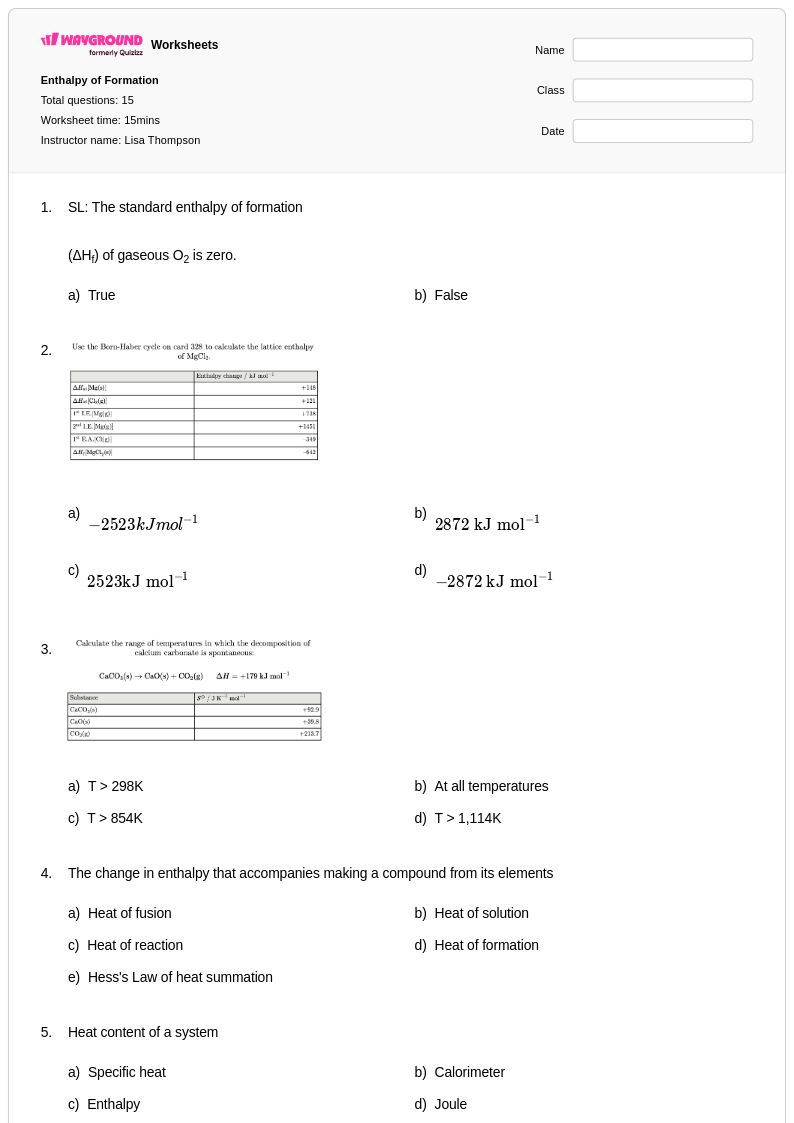
15 Q
12th - Uni

15 Q
12th - Uni

25 Q
12th - Uni

30 Q
8th

15 Q
12th - Uni

15 Q
12th - Uni

10 Q
11th
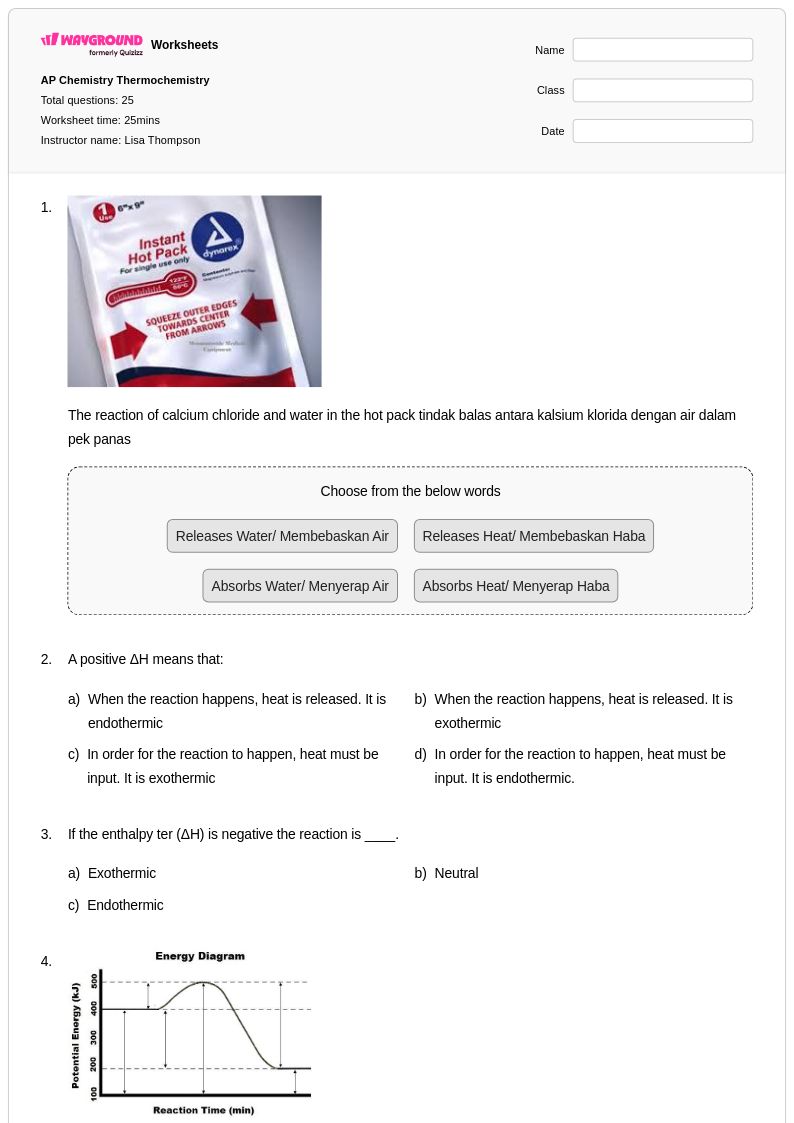
25 Q
10th - Uni
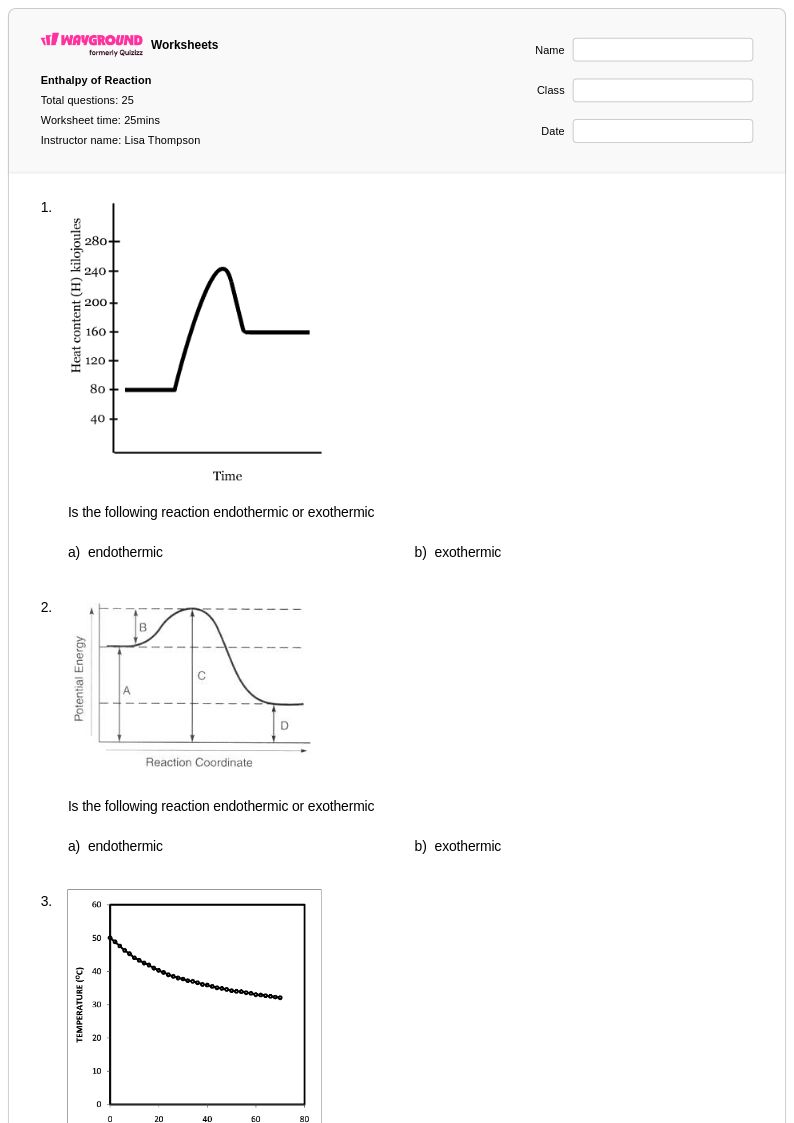
25 Q
12th - Uni

25 Q
10th - Uni

15 Q
12th - Uni

14 Q
9th
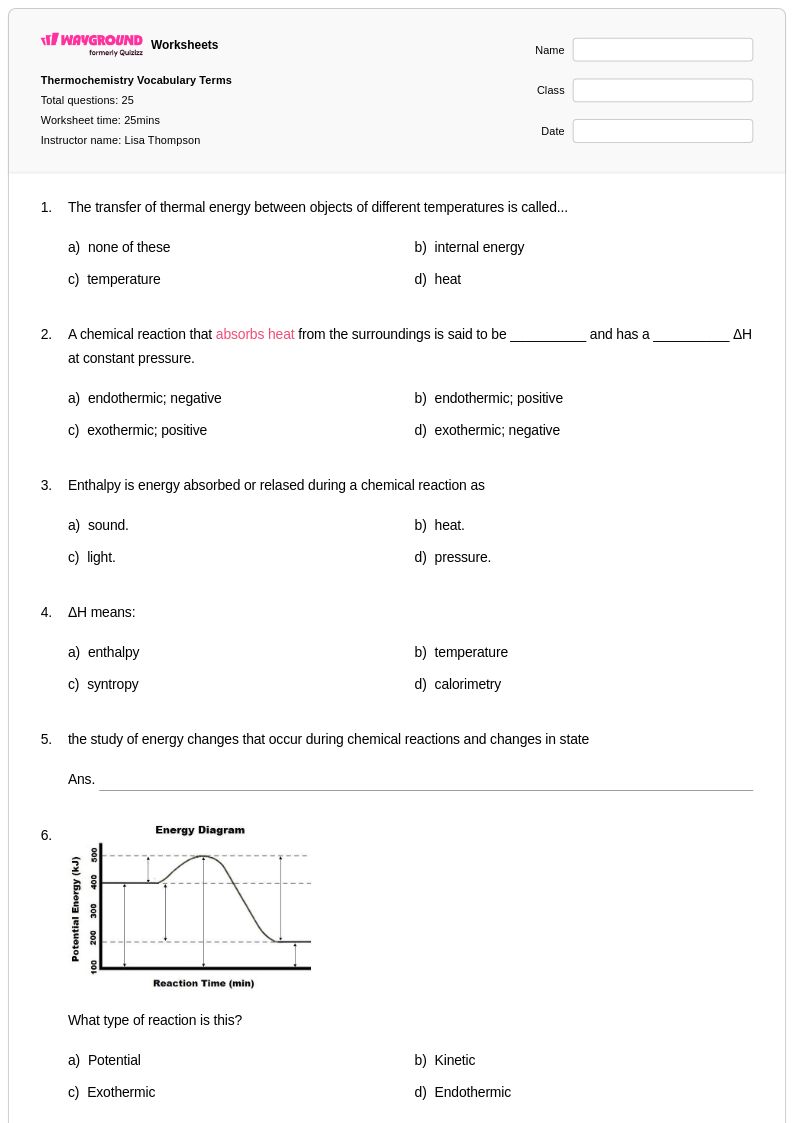
25 Q
10th - Uni

15 Q
10th - Uni
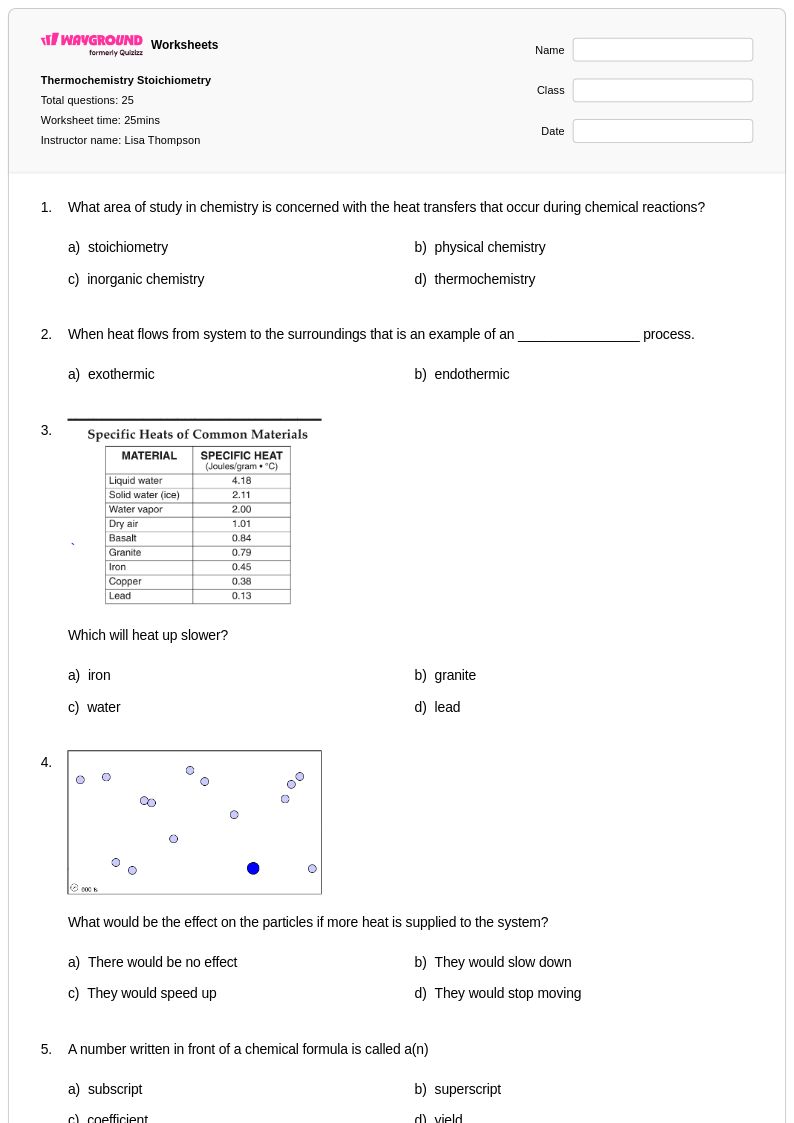
25 Q
10th - Uni
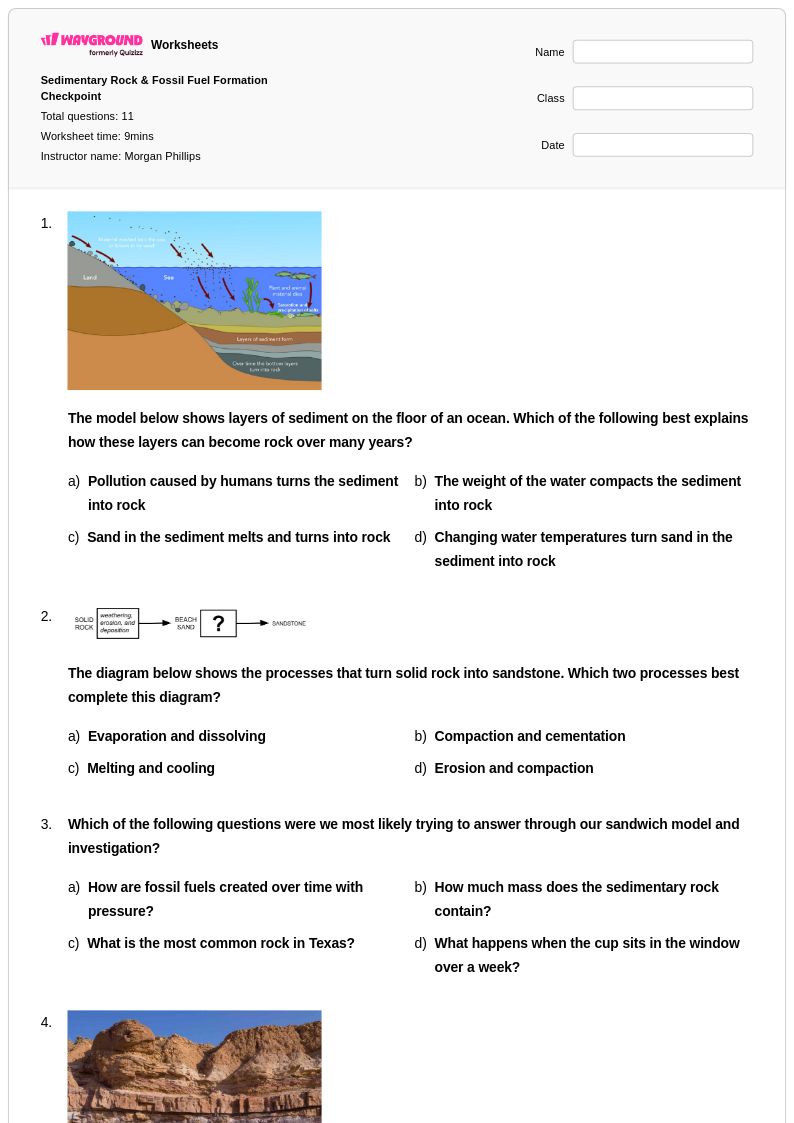
11 Q
5th
22 Q
5th

25 Q
8th - Uni

15 Q
10th - 12th

11 Q
9th - 12th

15 Q
6th - 8th

10 Q
5th - 6th

15 Q
6th - 8th

12 Q
5th - 6th
Explore Worksheets by Subjects
Explore printable Enthalpy of Formation worksheets
Enthalpy of formation worksheets available through Wayground (formerly Quizizz) provide comprehensive practice materials that help students master this fundamental thermochemistry concept. These carefully designed resources focus on calculating standard enthalpies of formation, interpreting thermodynamic data tables, and applying Hess's law to determine energy changes in chemical reactions. Students work through practice problems that strengthen their ability to analyze bond formation and breaking processes, calculate heat released or absorbed during reactions, and understand the relationship between molecular structure and energy content. The worksheets include detailed answer keys that guide students through step-by-step solutions, while printable pdf formats ensure accessibility for both classroom instruction and independent study sessions.
Wayground (formerly Quizizz) supports chemistry educators with millions of teacher-created enthalpy of formation resources that can be easily searched, filtered, and customized to meet diverse classroom needs. The platform's robust collection includes worksheets aligned with chemistry standards, offering teachers flexible options for differentiated instruction that accommodates varying student skill levels and learning preferences. These digital and printable materials streamline lesson planning by providing ready-to-use content for skill practice, remediation sessions, and enrichment activities. Teachers can modify existing worksheets or combine multiple resources to create comprehensive assessment tools, while the platform's organizational features help educators efficiently locate specific problem types, from basic enthalpy calculations to complex multi-step thermodynamic analyses that prepare students for advanced chemistry coursework.
