
15 Q
12th - Uni
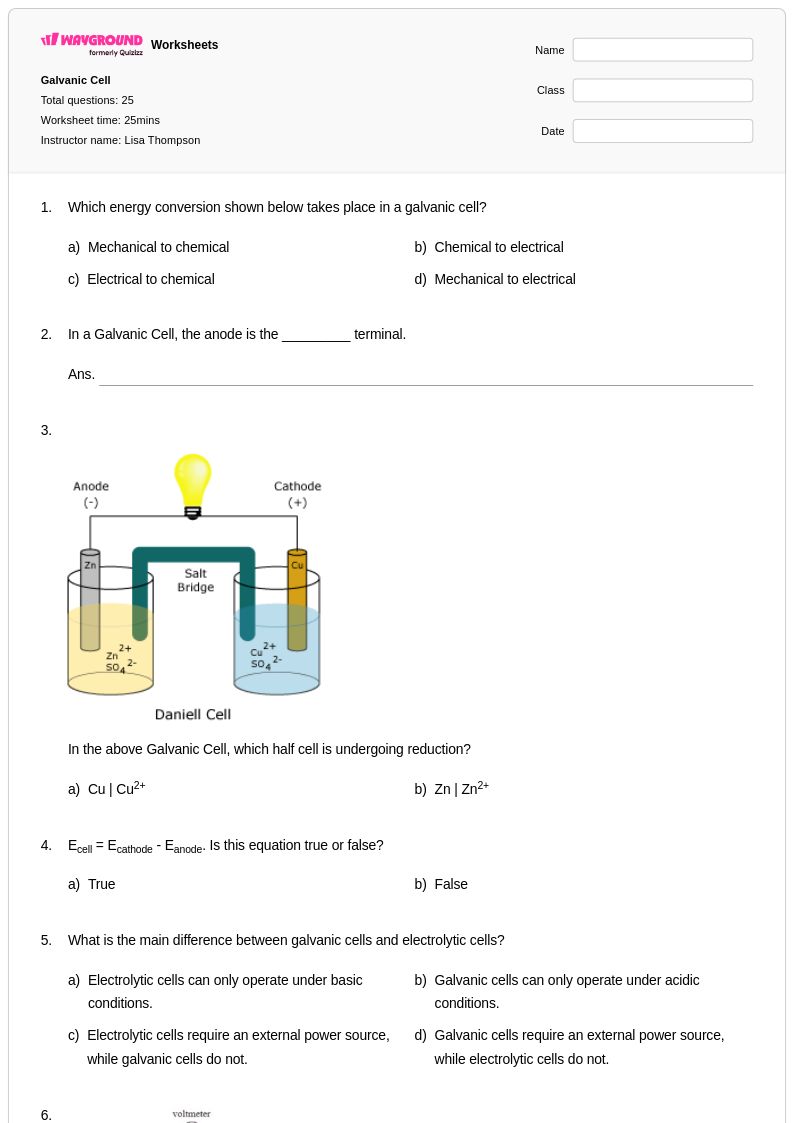
25 Q
12th - Uni

19 Q
10th

15 Q
12th - Uni

11 Q
KG
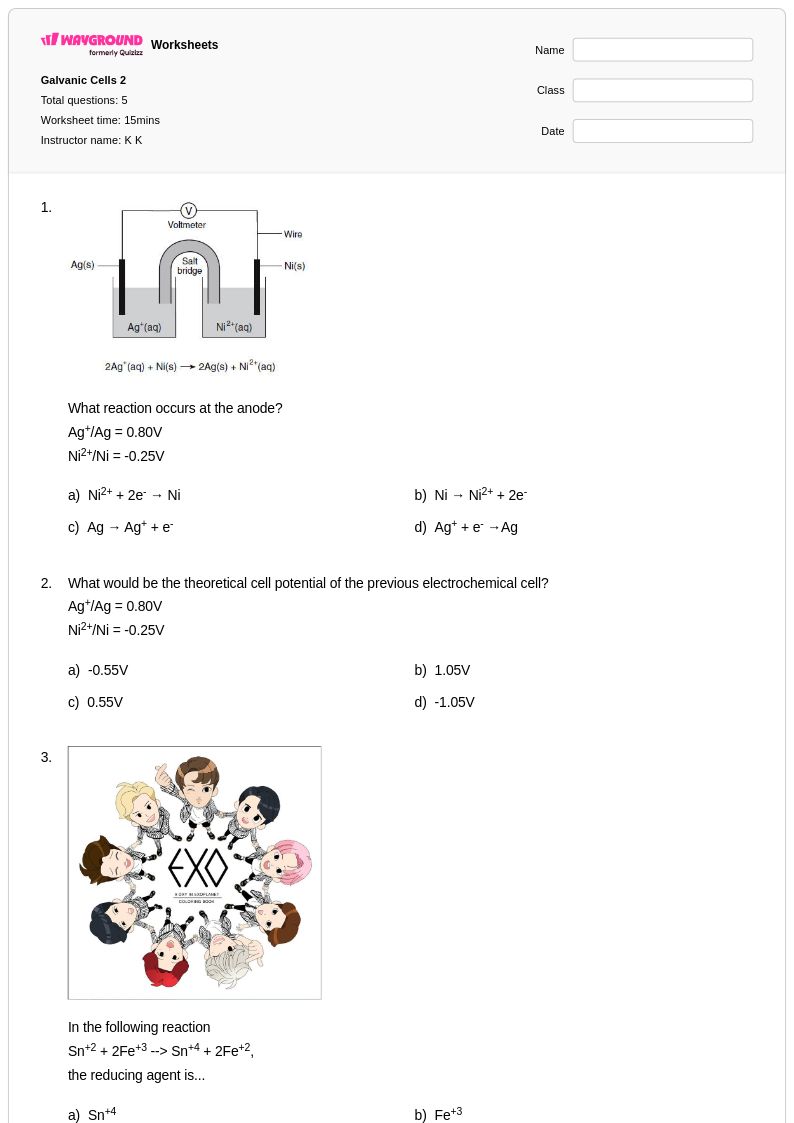
5 Q
KG
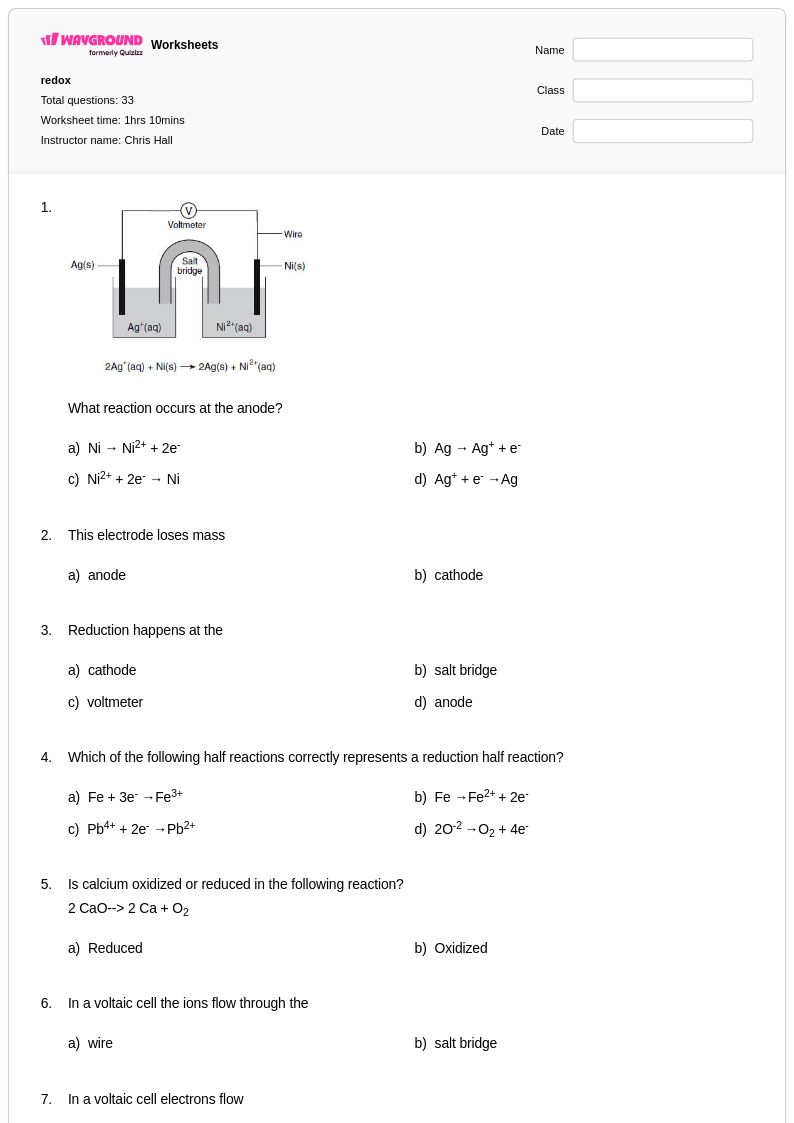
33 Q
12th
11 Q
6th

25 Q
7th
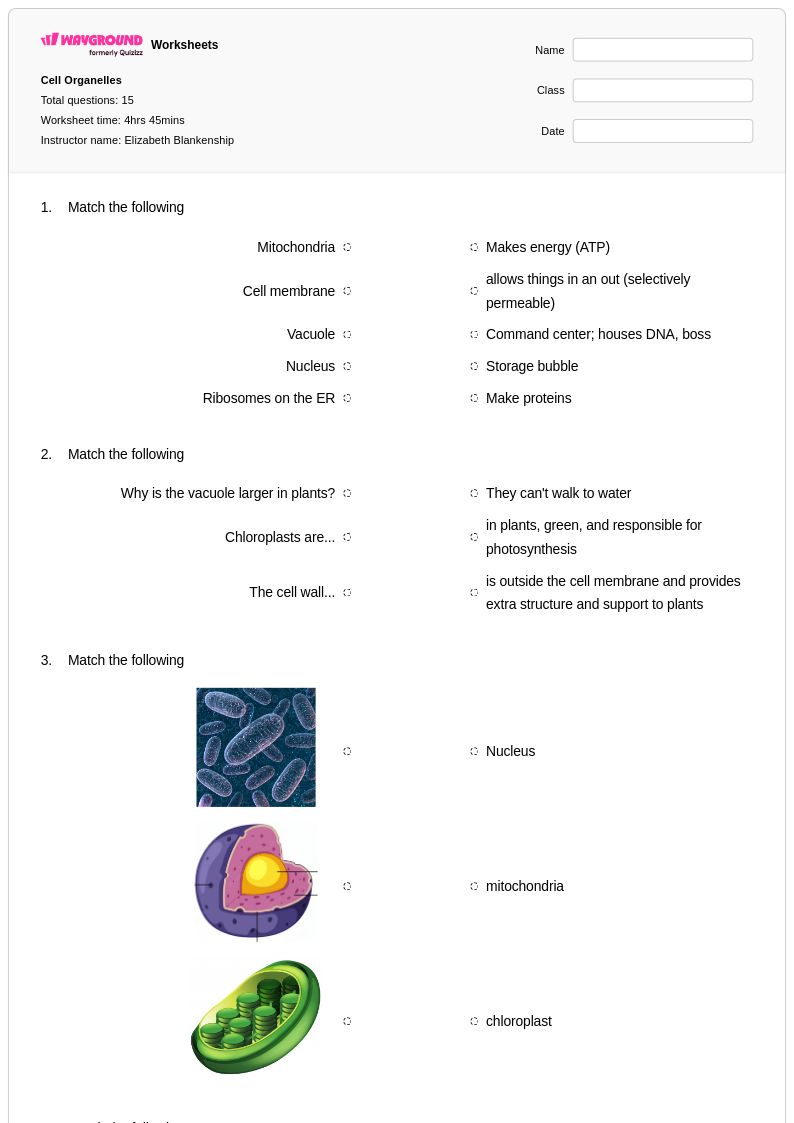
15 Q
8th
20 Q
6th
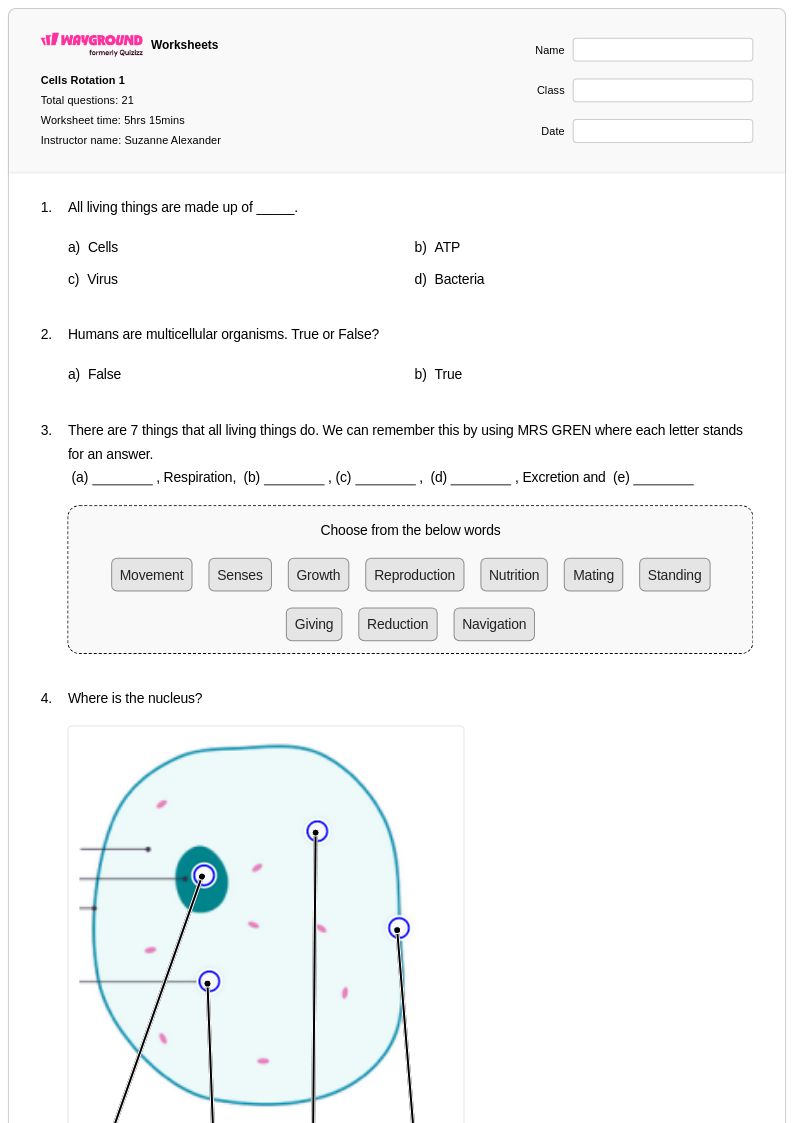
21 Q
7th
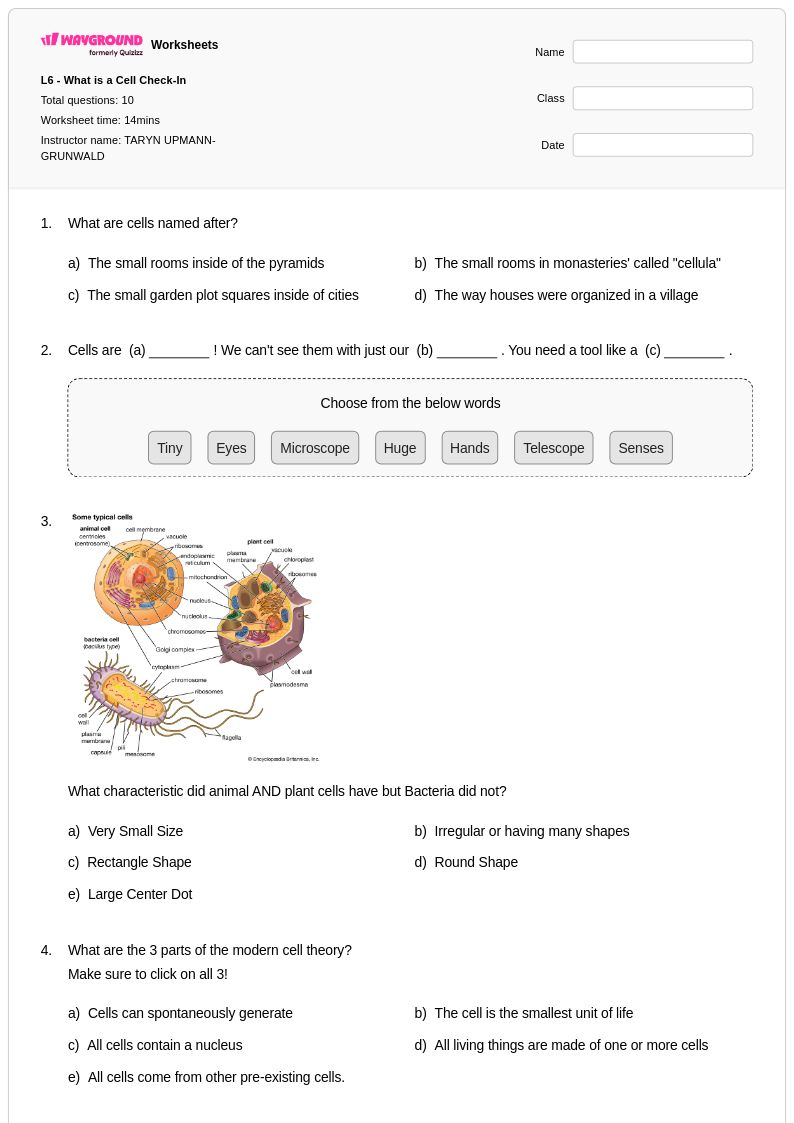
10 Q
7th

14 Q
7th
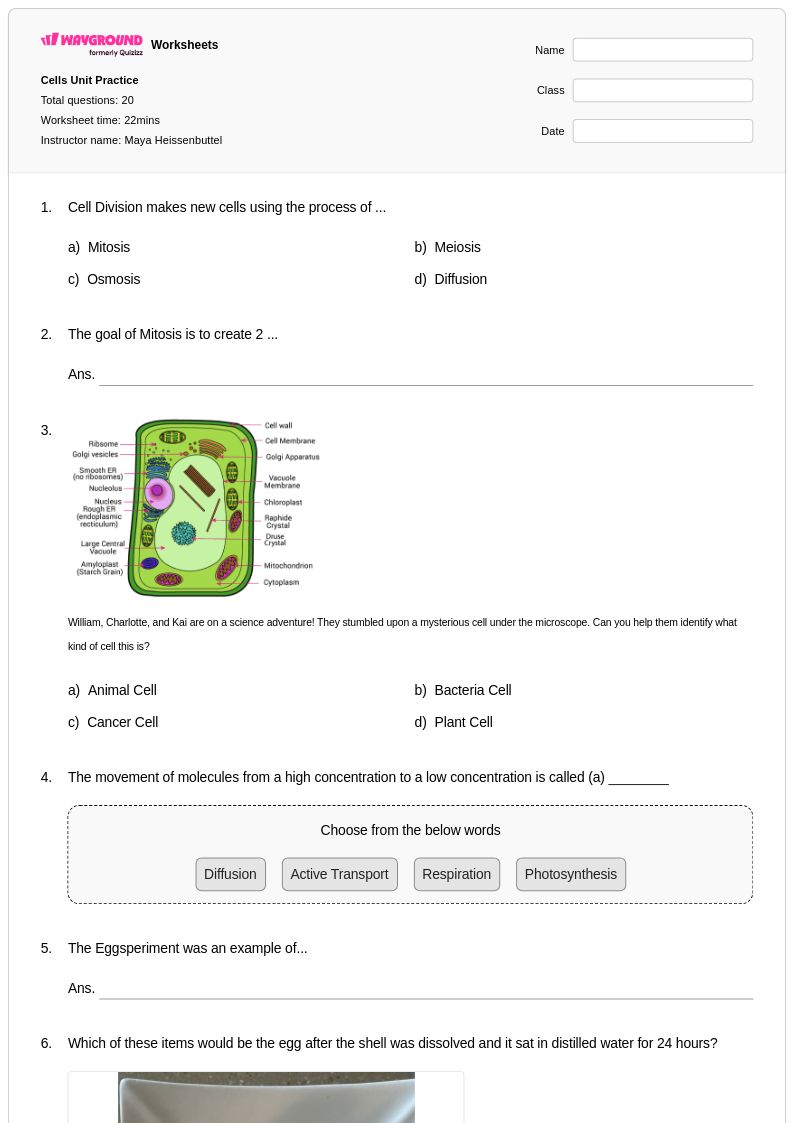
20 Q
7th
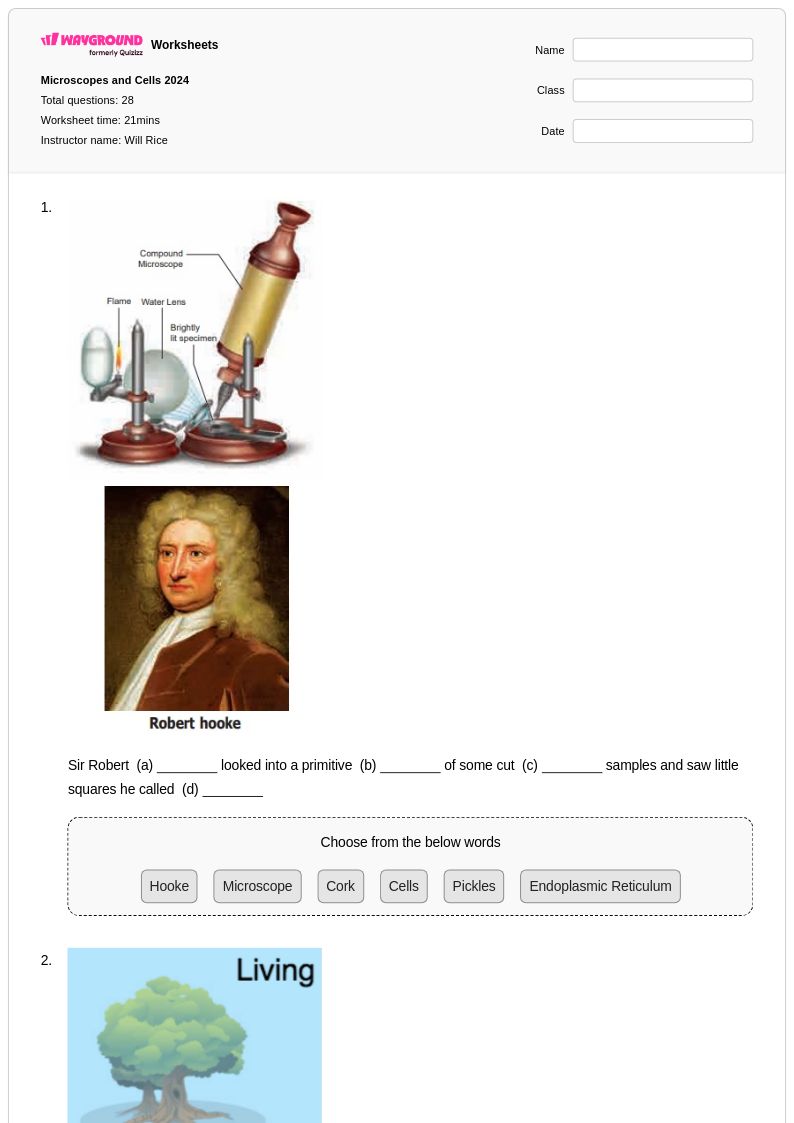
28 Q
7th

10 Q
7th
14 Q
10th
20 Q
7th
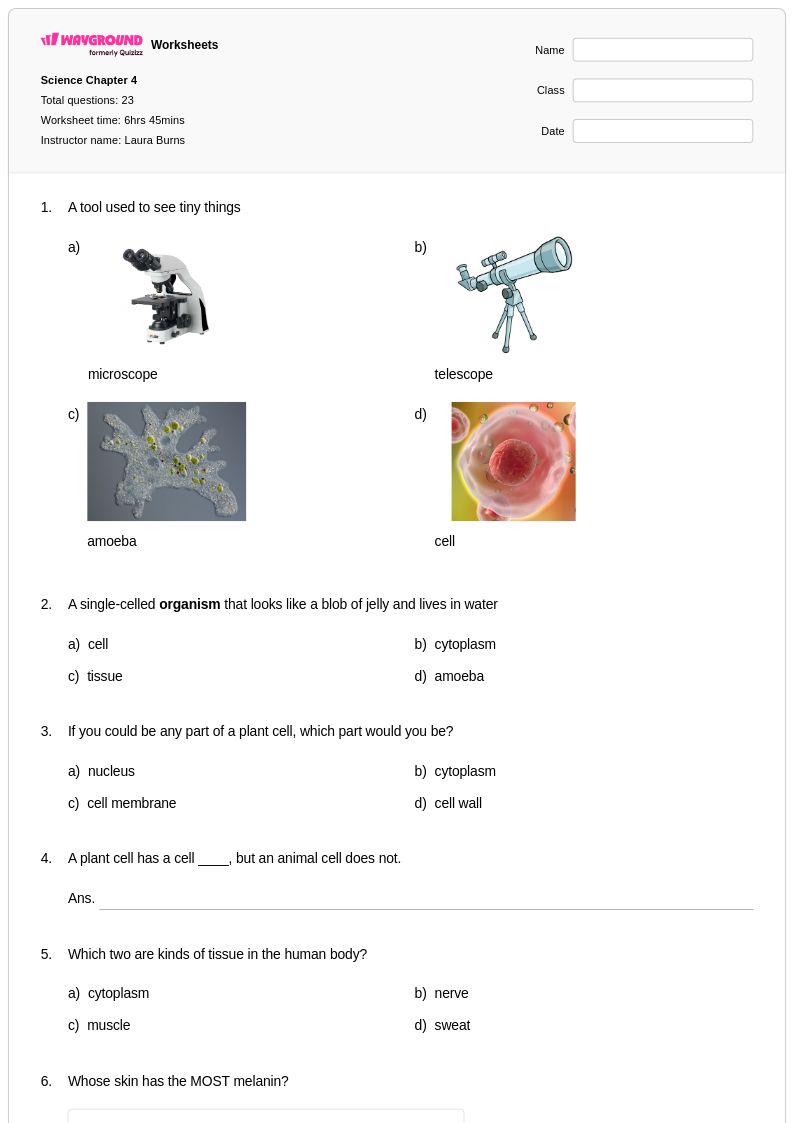
23 Q
3rd

30 Q
6th
21 Q
9th

16 Q
9th
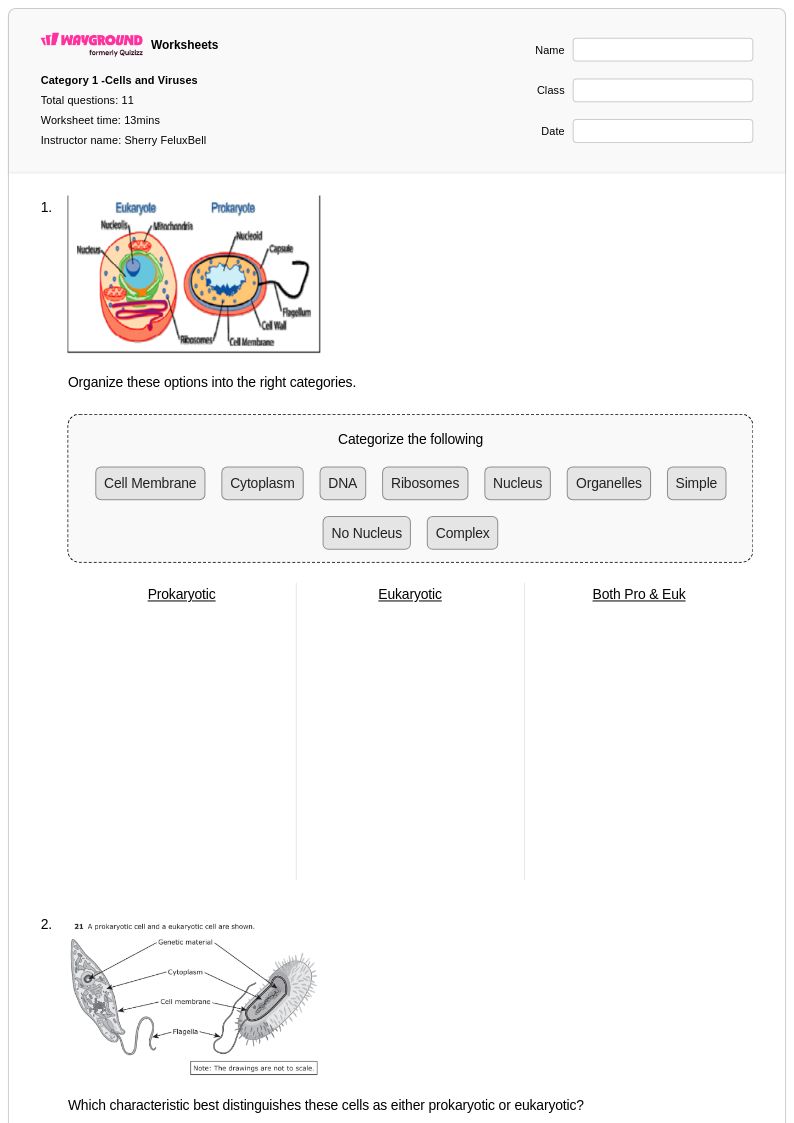
11 Q
9th
Explore Worksheets by Subjects
Explore printable Galvanic Cell worksheets
Galvanic cell worksheets available through Wayground (formerly Quizizz) provide comprehensive practice materials that help students master the fundamental principles of electrochemistry and spontaneous redox reactions. These expertly designed resources focus on building critical skills including identifying anode and cathode components, calculating cell potentials using standard reduction potentials, writing balanced half-reactions, and understanding electron flow in electrochemical cells. Students work through practice problems that cover salt bridge functions, galvanic cell diagrams, and the relationship between Gibbs free energy and cell voltage. The worksheets include detailed answer keys that support independent learning and self-assessment, while the free printable pdf format ensures easy classroom distribution and homework assignments.
Wayground (formerly Quizizz) empowers science teachers with millions of teacher-created galvanic cell resources that streamline lesson planning and enhance student understanding of electrochemistry concepts. The platform's robust search and filtering capabilities allow educators to quickly locate materials aligned with specific chemistry standards and learning objectives, while built-in differentiation tools enable customization for varying skill levels within the classroom. Teachers can access these comprehensive worksheet collections in both printable and digital pdf formats, facilitating seamless integration into hybrid learning environments and supporting diverse instructional approaches. These versatile resources prove invaluable for targeted remediation of challenging electrochemistry concepts, enrichment activities for advanced learners, and systematic skill practice that reinforces understanding of galvanic cell operations and calculations.
