
20 Q
10th

20 Q
9th - 12th

9 Q
11th

20 Q
10th
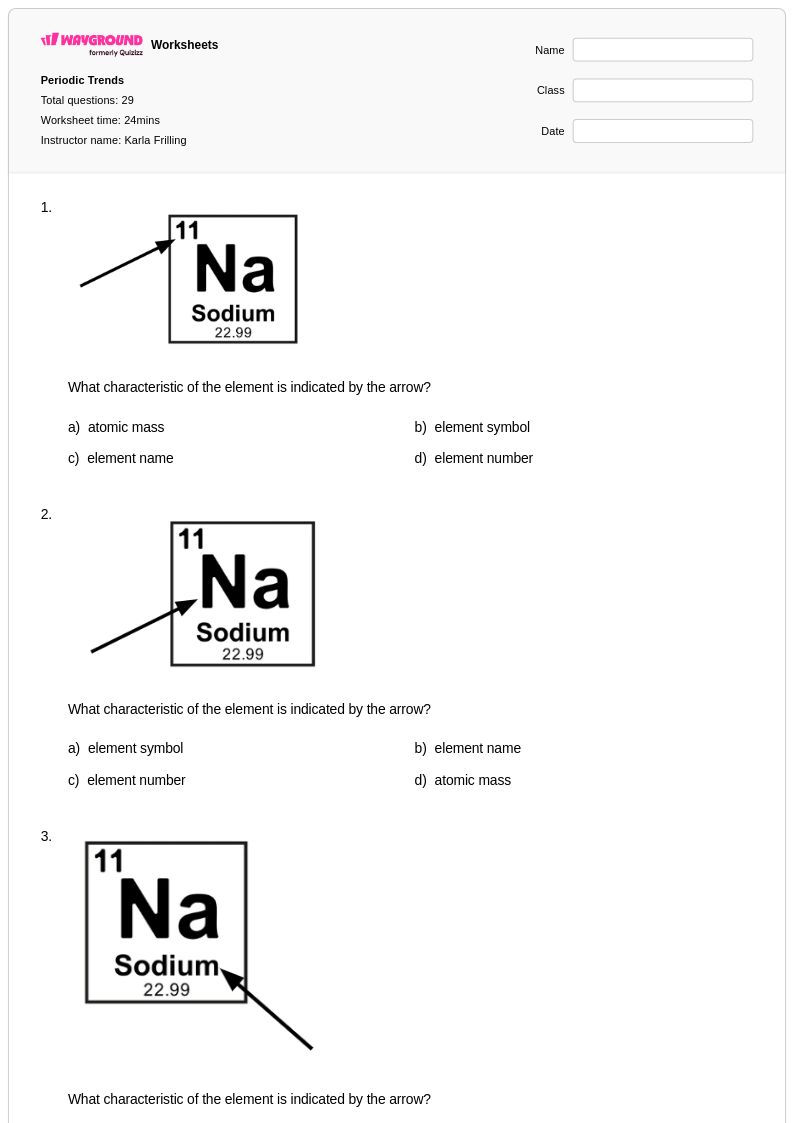
29 Q
7th

10 Q
10th - 12th
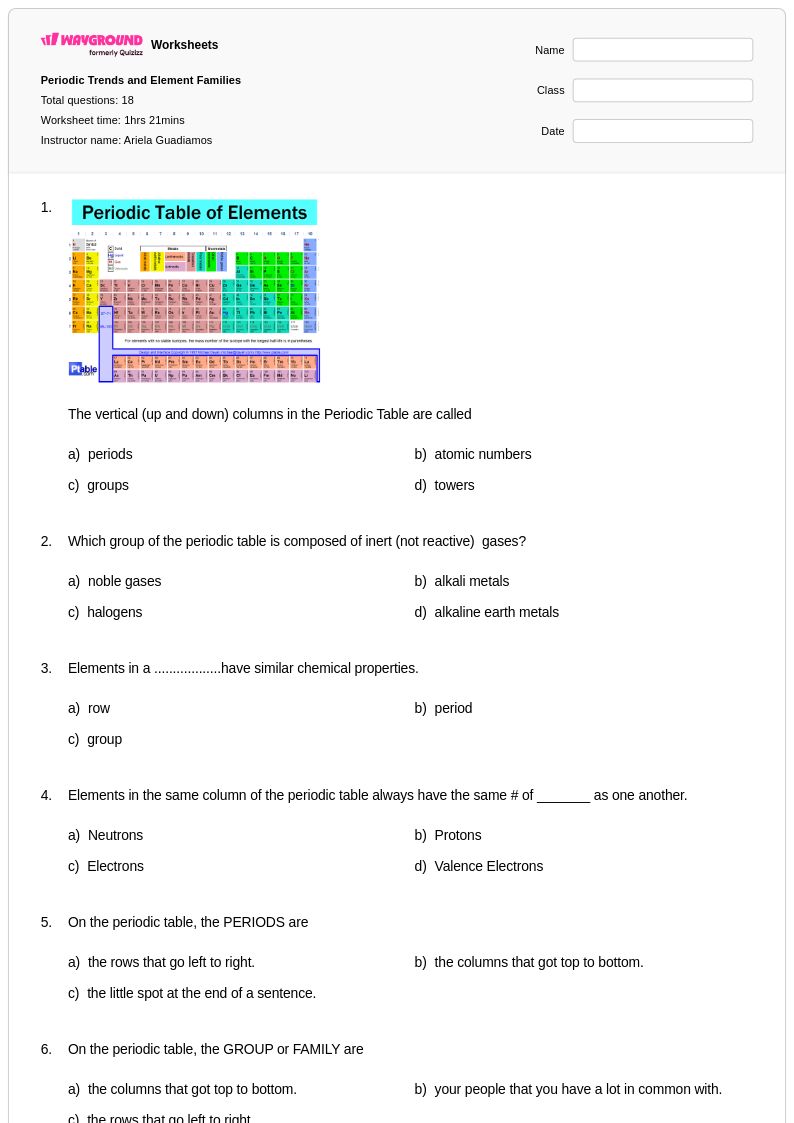
18 Q
7th

10 Q
9th - 12th

20 Q
10th

37 Q
10th

24 Q
11th

38 Q
9th - 12th
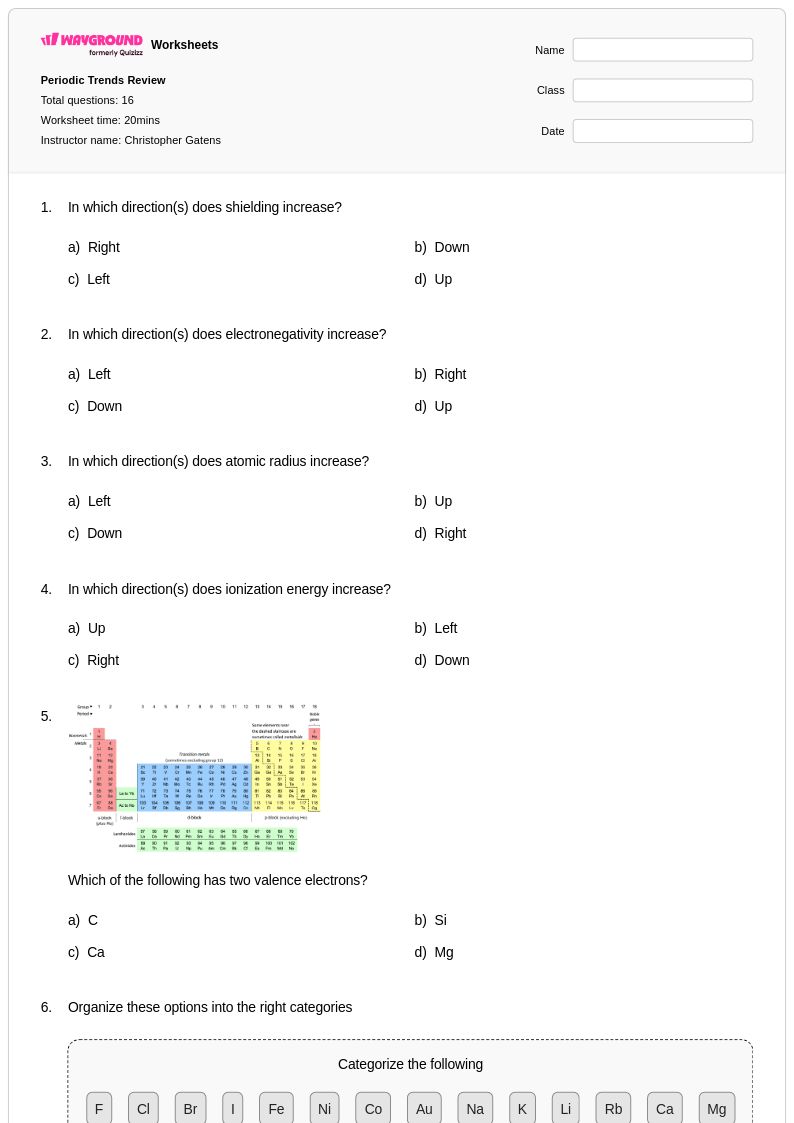
16 Q
9th - 12th

21 Q
10th
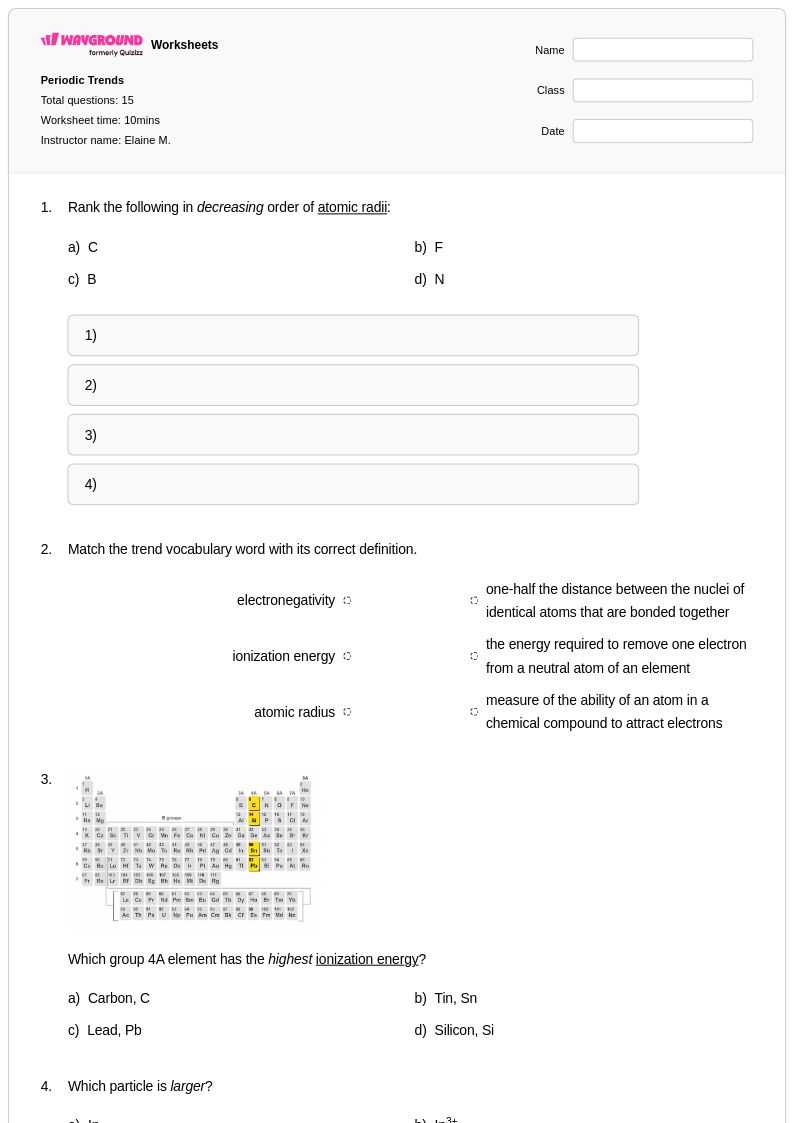
15 Q
10th - 12th

22 Q
9th - 12th

47 Q
10th

25 Q
10th

20 Q
8th

20 Q
10th
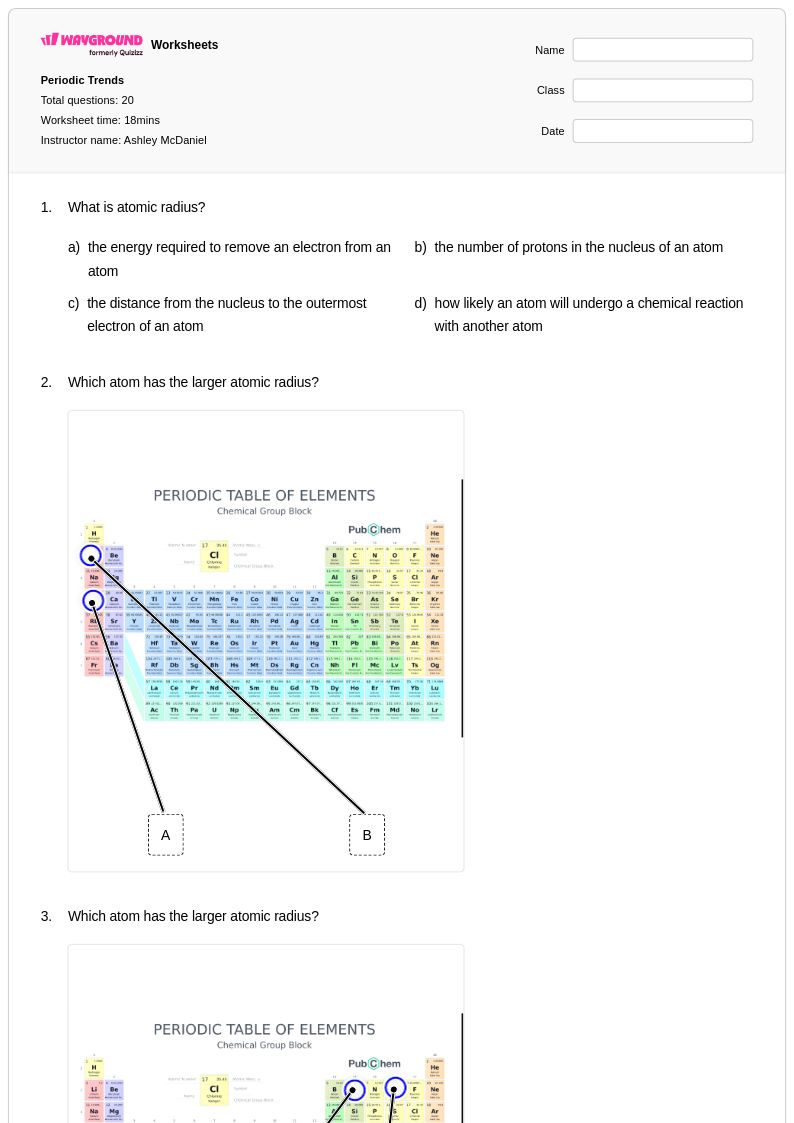
20 Q
9th
32 Q
9th

30 Q
11th

20 Q
9th - 12th
Explore Worksheets by Subjects
Explore printable Periodic Trends worksheets
Periodic trends worksheets available through Wayground (formerly Quizizz) provide comprehensive practice materials that help students master the predictable patterns found across the periodic table of elements. These chemistry resources focus on developing critical analytical skills as students explore how atomic radius, ionization energy, electronegativity, and metallic character change systematically across periods and down groups. The worksheets feature carefully structured practice problems that guide learners through interpreting periodic trends using electron configuration principles and effective nuclear charge concepts. Each printable resource includes detailed answer keys that support independent study and self-assessment, while the free pdf format ensures easy classroom distribution and homework assignments that reinforce fundamental chemistry concepts.
Wayground (formerly Quizizz) empowers chemistry educators with an extensive collection of millions of teacher-created periodic trends resources that streamline lesson planning and differentiated instruction. The platform's robust search and filtering capabilities allow teachers to quickly locate worksheets that align with specific chemistry standards and match their students' varying skill levels. These customizable materials support both remediation for struggling learners and enrichment opportunities for advanced students, with flexible formatting options that accommodate both digital classroom environments and traditional printable assignments. The comprehensive worksheet collections enable teachers to provide targeted skill practice that builds conceptual understanding of periodic law applications, electron shielding effects, and the relationship between atomic structure and chemical properties across all elements in the periodic table.
