25 Q
11th
20 Q
10th

12 Q
10th - 12th
20 Q
9th - 12th

6 Q
11th

25 Q
10th - Uni

18 Q
Uni
15 Q
11th - Uni
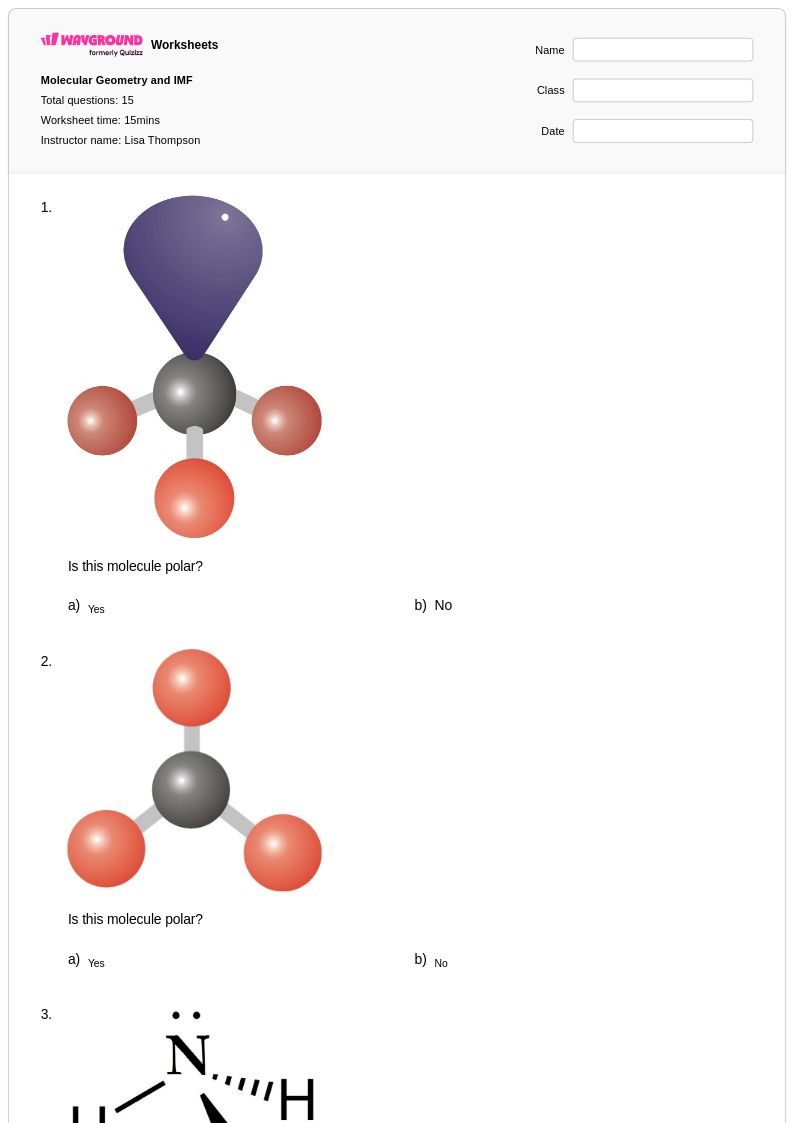
15 Q
11th - Uni
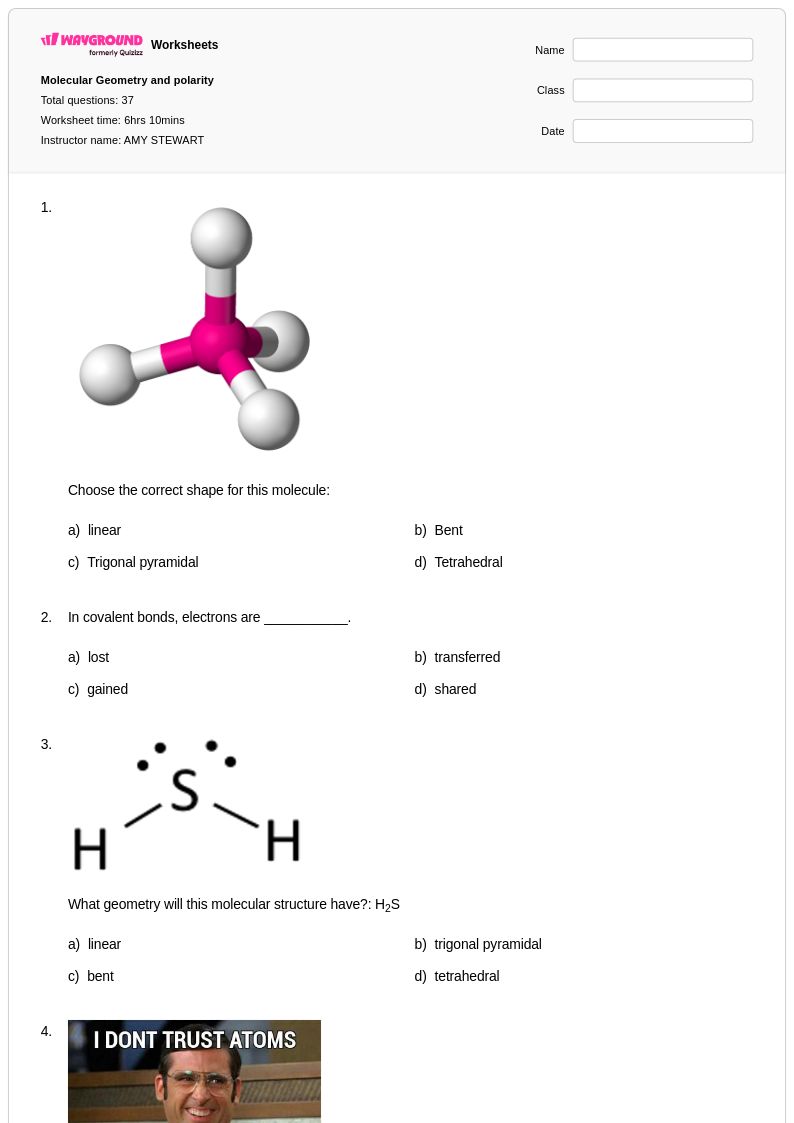
37 Q
10th
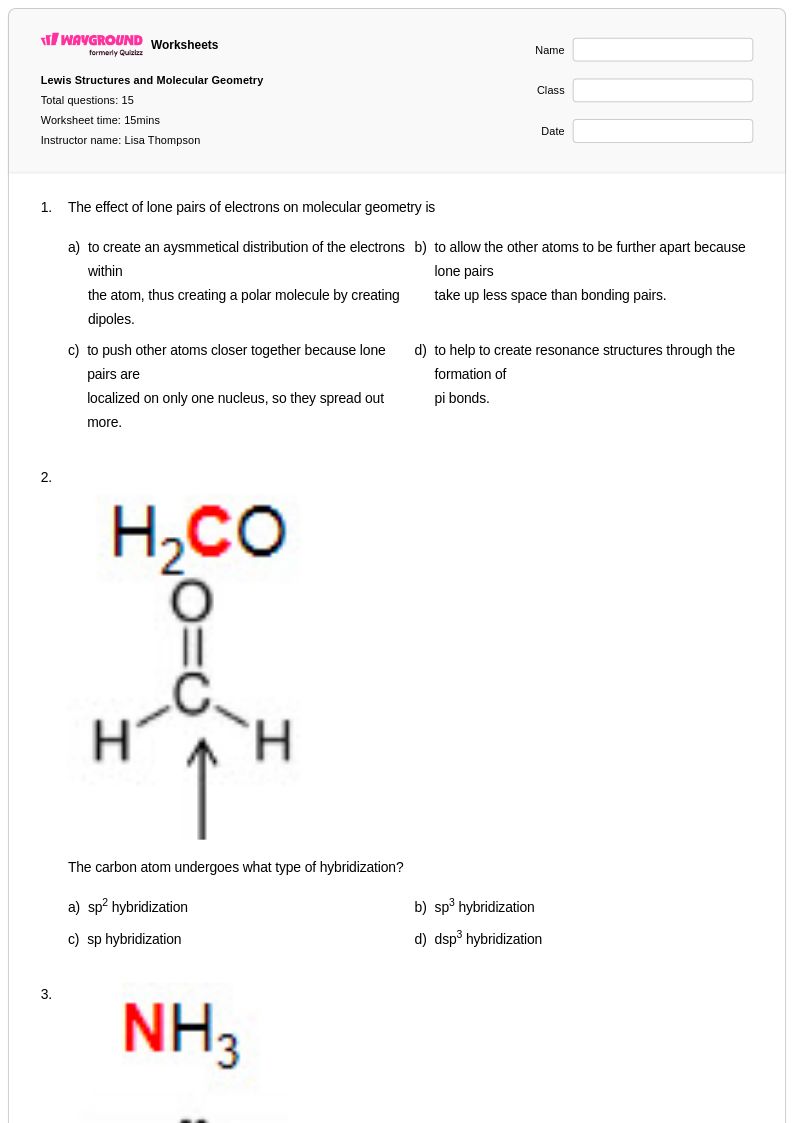
15 Q
11th - Uni
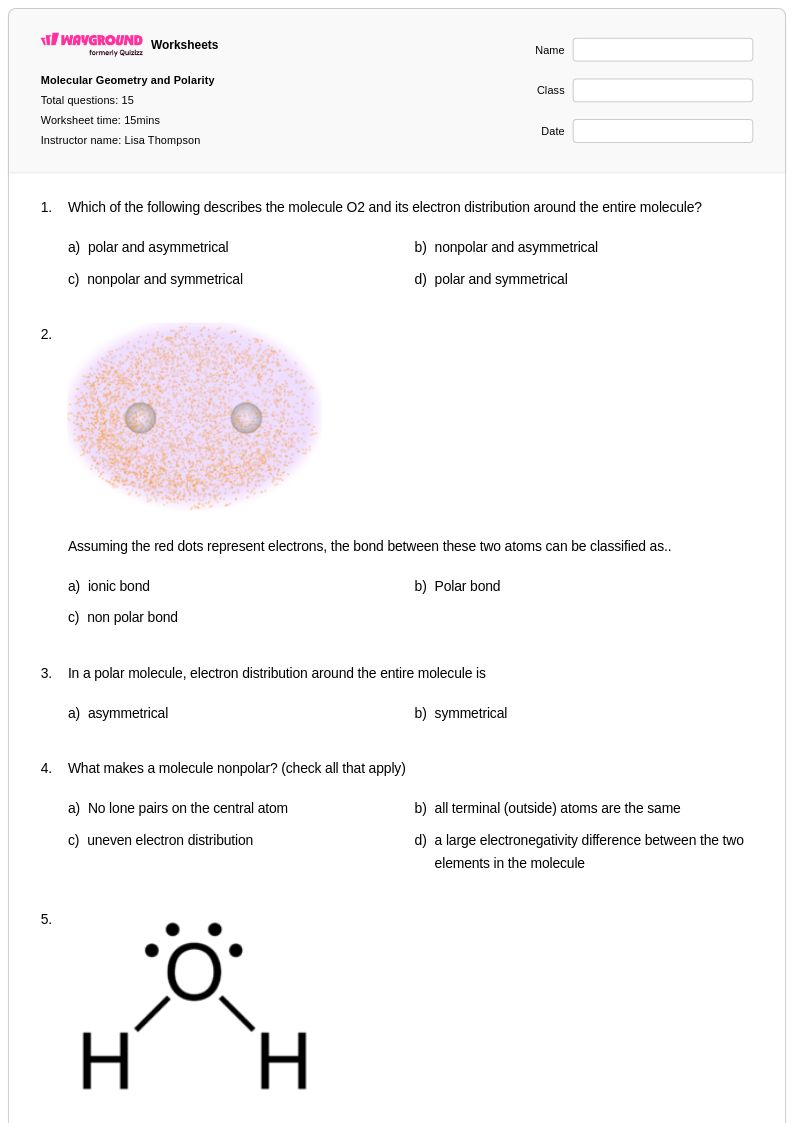
15 Q
11th - Uni
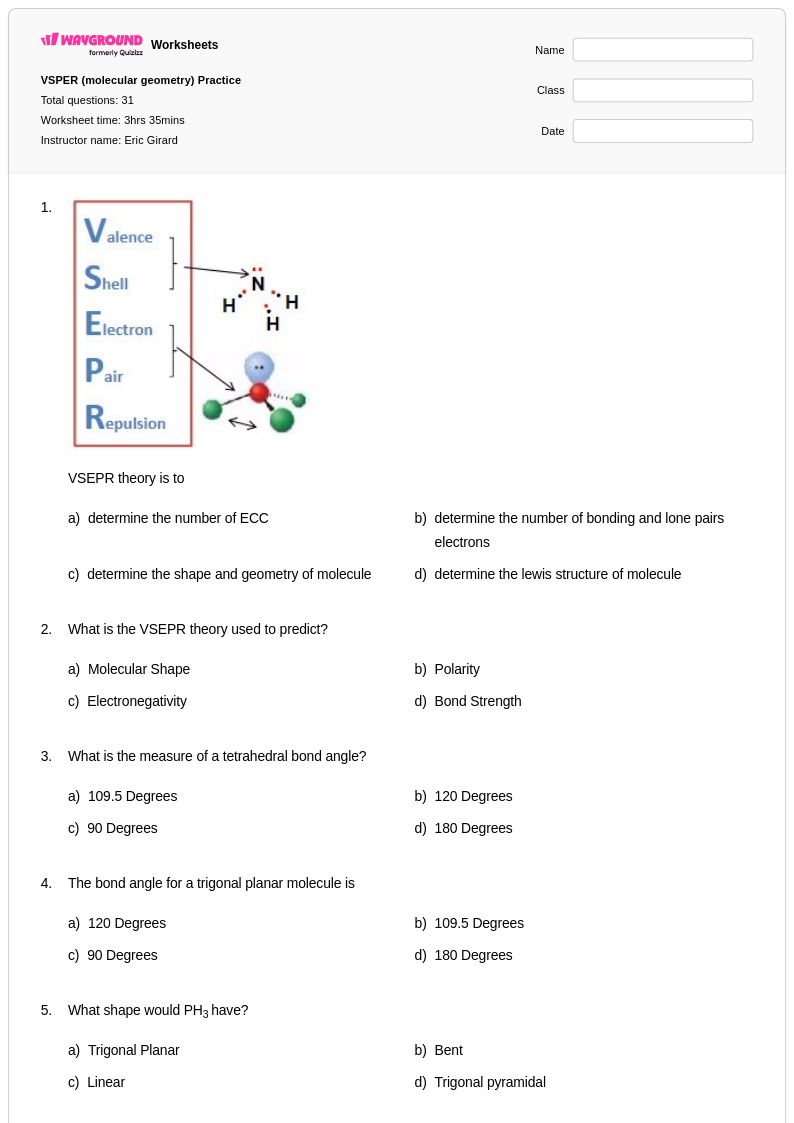
31 Q
12th
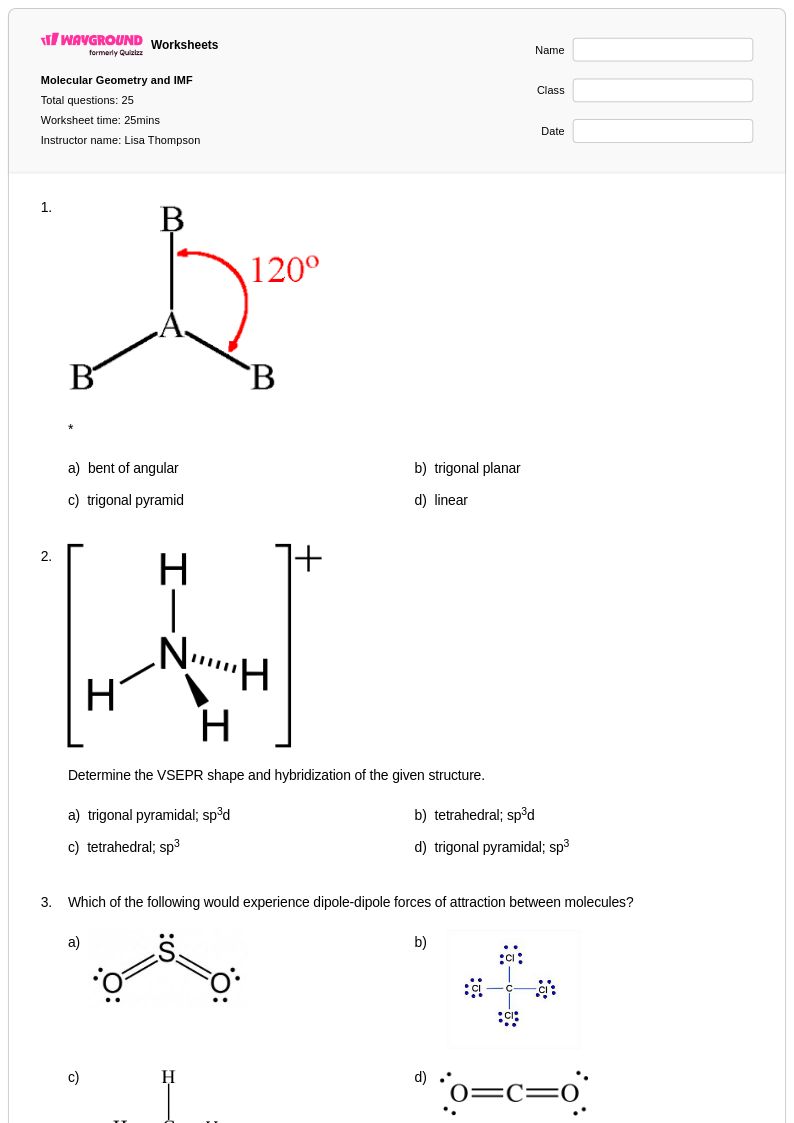
25 Q
11th - Uni
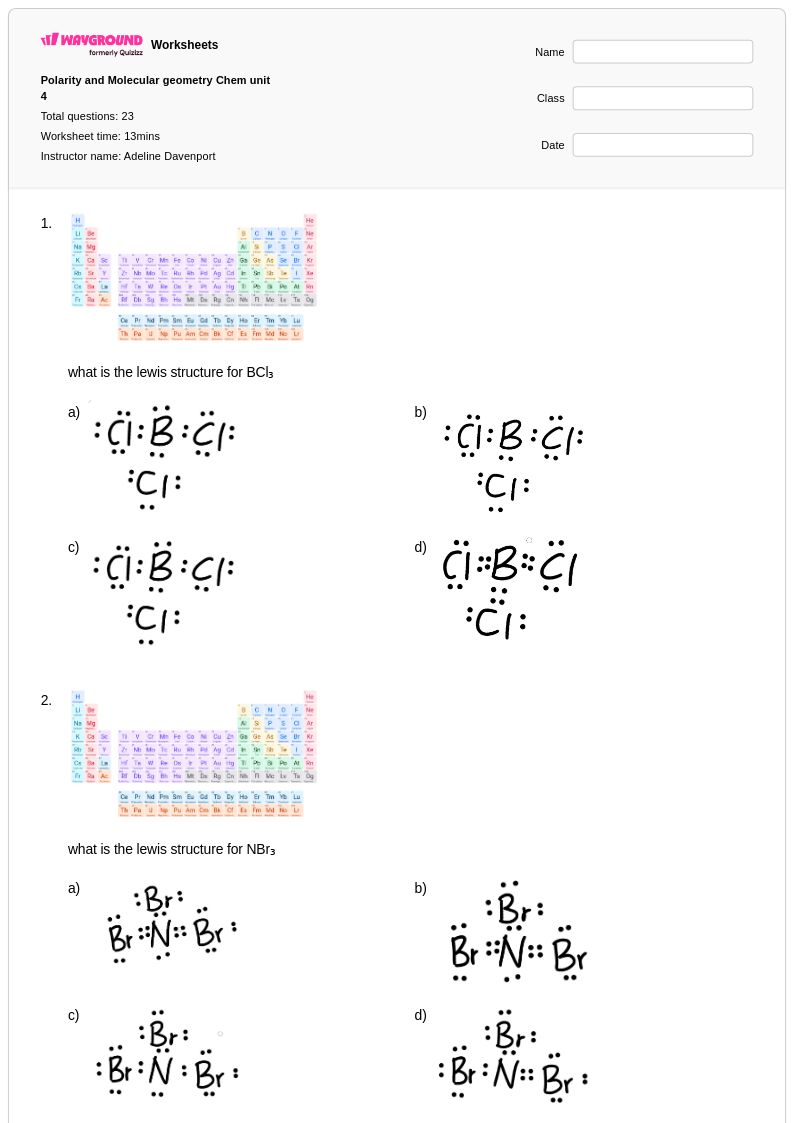
23 Q
10th

25 Q
11th - Uni

100 Q
12th
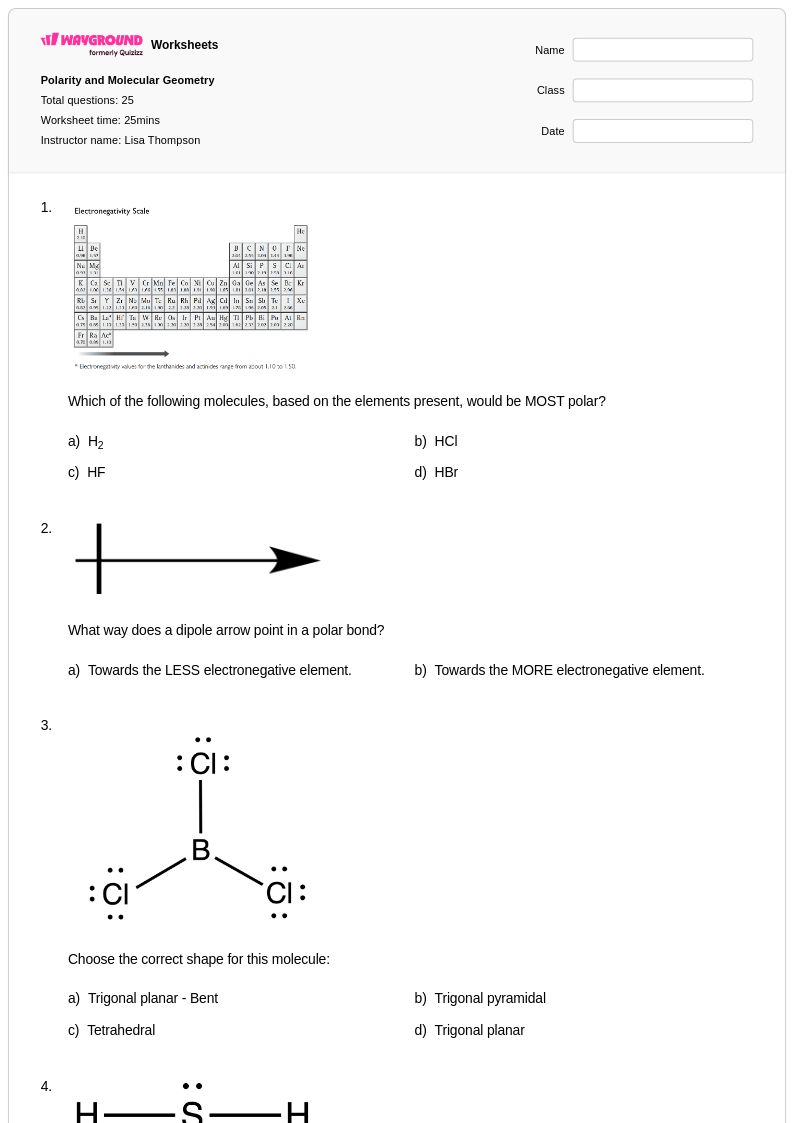
25 Q
11th - Uni
30 Q
8th - 12th
26 Q
9th - 12th
31 Q
10th
11 Q
9th - 12th
42 Q
9th - 12th

8 Q
12th
Explore Worksheets by Subjects
Explore printable Molecular Geometry worksheets
Molecular geometry worksheets available through Wayground (formerly Quizizz) provide comprehensive practice materials that help students master the three-dimensional arrangements of atoms within molecules. These expertly crafted worksheets strengthen essential chemistry skills including VSEPR theory application, bond angle prediction, electron pair geometry identification, and molecular shape determination. Students work through practice problems that cover linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral geometries, while developing spatial visualization abilities crucial for understanding chemical bonding and molecular behavior. Each worksheet collection includes detailed answer keys and is available as free printable pdf resources, allowing students to systematically build their understanding of how electron pair repulsion influences molecular architecture.
Wayground (formerly Quizizz) empowers chemistry teachers with millions of teacher-created molecular geometry resources that feature robust search and filtering capabilities aligned to educational standards. The platform's differentiation tools enable educators to customize worksheets based on individual student needs, offering both printable pdf formats for traditional classroom use and digital versions for interactive learning environments. These flexible molecular geometry collections support comprehensive lesson planning while providing targeted materials for remediation of struggling students and enrichment opportunities for advanced learners. Teachers can efficiently locate resources that match specific curriculum requirements, from basic Lewis structure interpretation to complex hybridization concepts, ensuring consistent skill practice and assessment across diverse learning objectives in chemistry education.
