
20 Q
8th
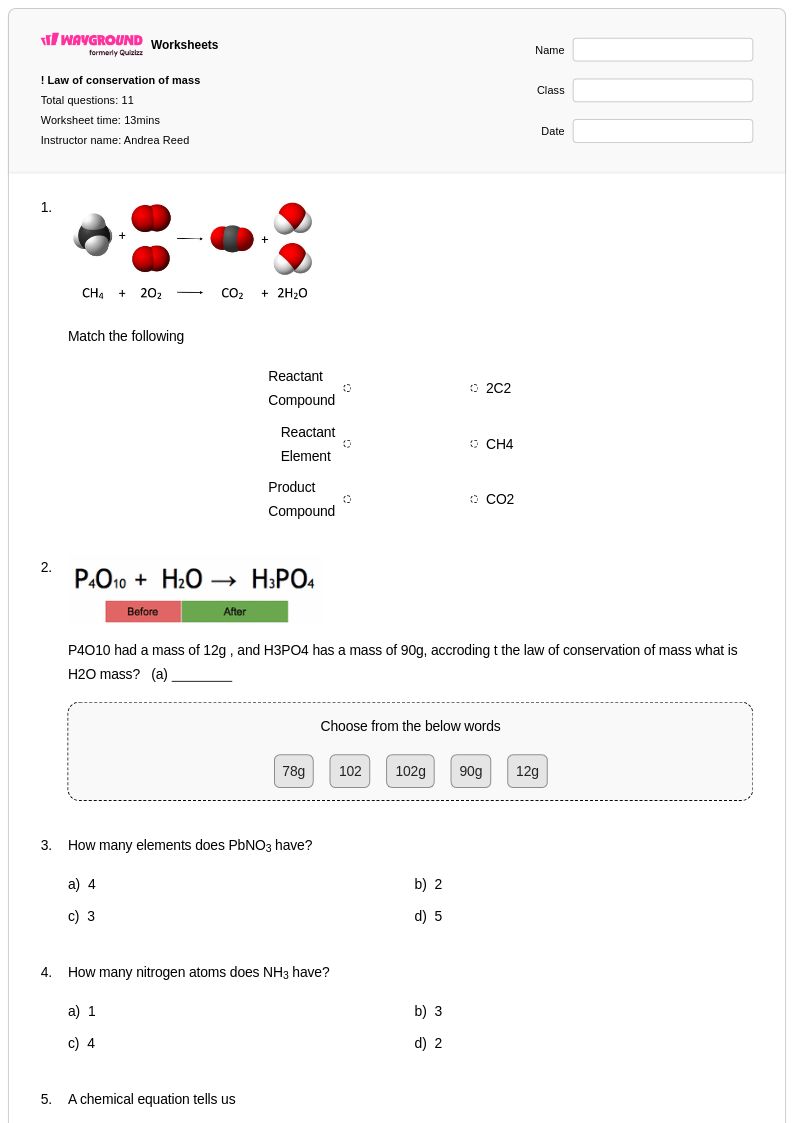
11 Q
8th

10 Q
8th

22 Q
9th
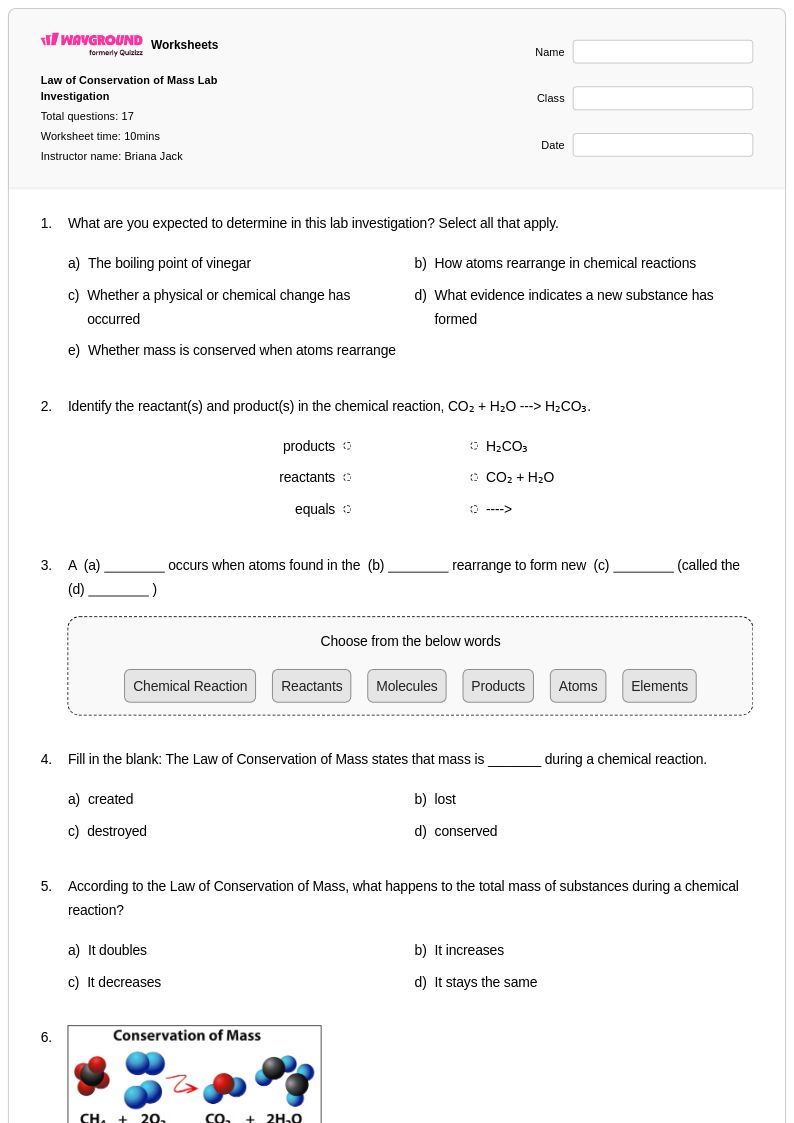
17 Q
8th

20 Q
7th
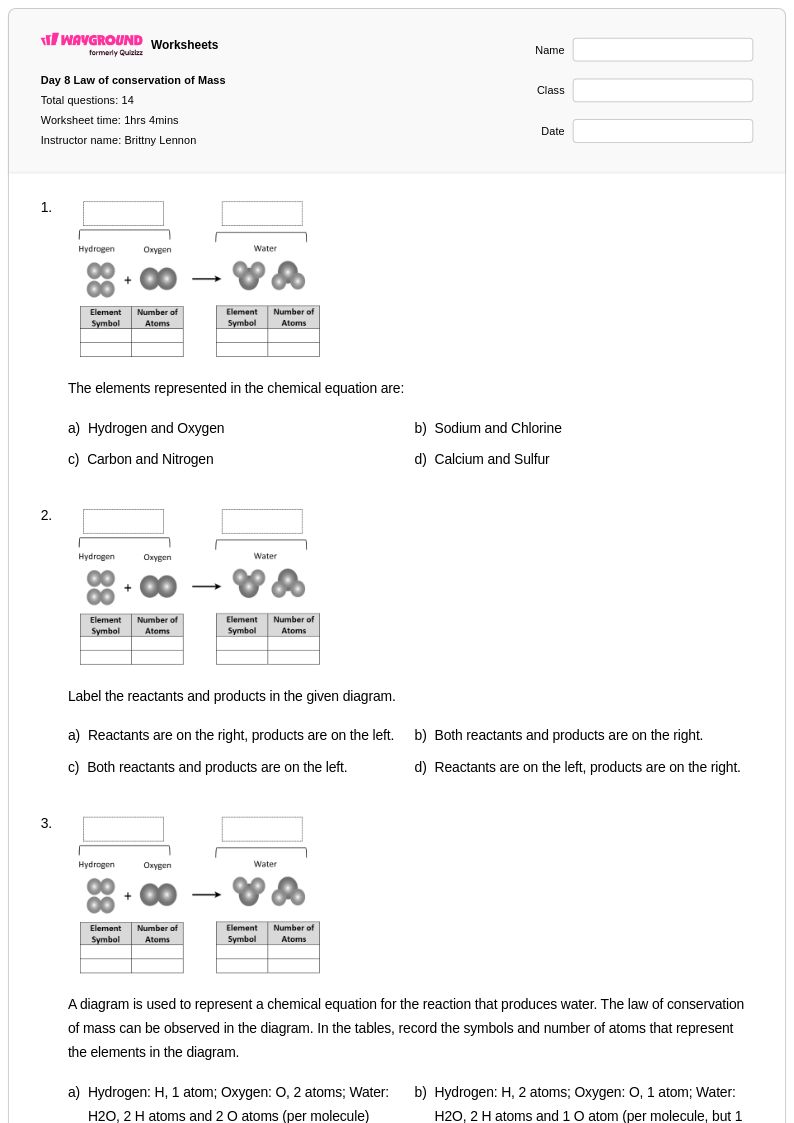
14 Q
8th

56 Q
6th - 8th

15 Q
7th

12 Q
8th

18 Q
KG - 1st

30 Q
7th
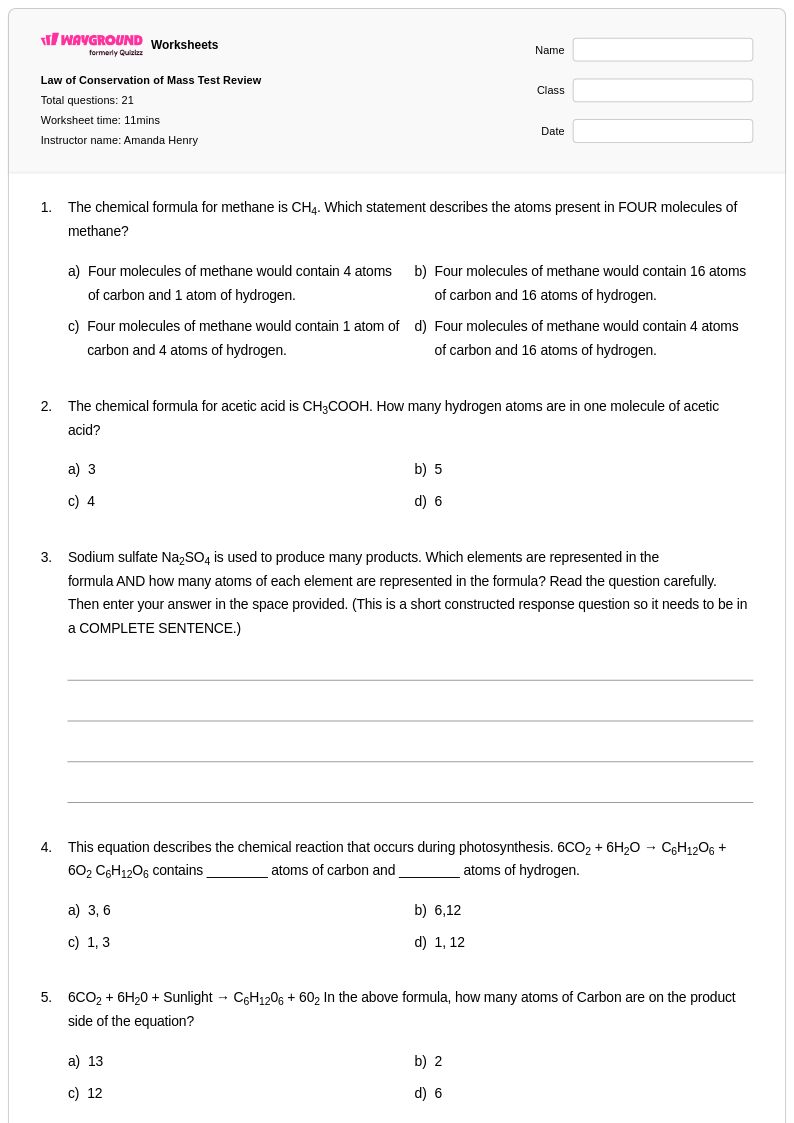
21 Q
6th - 8th
11 Q
6th
25 Q
8th - Uni

21 Q
7th

6 Q
8th
13 Q
5th

14 Q
7th

25 Q
7th - Uni

25 Q
8th
27 Q
7th

15 Q
9th - 12th

25 Q
8th - Uni
Explore Worksheets by Subjects
Explore printable Law of Conservation of Mass worksheets
Law of Conservation of Mass worksheets available through Wayground (formerly Quizizz) provide comprehensive practice opportunities for students to master this fundamental principle of chemistry. These educational resources focus on developing critical thinking skills around mass relationships in chemical reactions, helping students understand that matter cannot be created or destroyed during chemical processes. The worksheets feature diverse practice problems that challenge students to balance chemical equations, calculate reactant and product masses, and analyze experimental data to verify conservation principles. Each worksheet collection includes detailed answer keys and is available as free printable PDFs, making it easy for educators to incorporate hands-on problem-solving activities that reinforce theoretical concepts through quantitative analysis and real-world applications.
Wayground (formerly Quizizz) empowers educators with access to millions of teacher-created Law of Conservation of Mass worksheet resources that support diverse classroom needs and learning objectives. The platform's robust search and filtering capabilities allow teachers to quickly locate materials aligned with specific chemistry standards and differentiate instruction based on student readiness levels. These customizable worksheet collections are available in both printable PDF formats and interactive digital versions, providing flexibility for various teaching environments and learning preferences. Teachers can leverage these comprehensive resources for lesson planning, targeted remediation of misconceptions about mass conservation, enrichment activities for advanced learners, and systematic skill practice that builds conceptual understanding through repeated exposure to balanced equation problems and stoichiometric calculations.
