
25 Q
11th - Uni
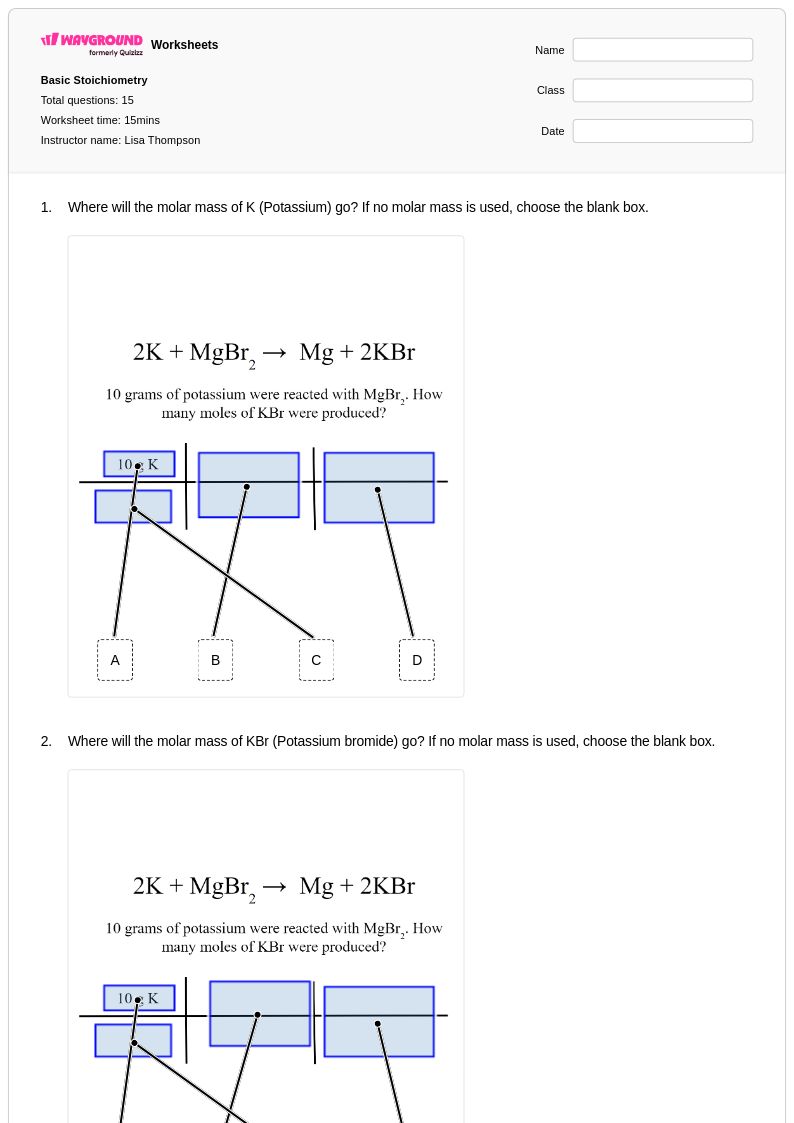
15 Q
10th - Uni

32 Q
9th
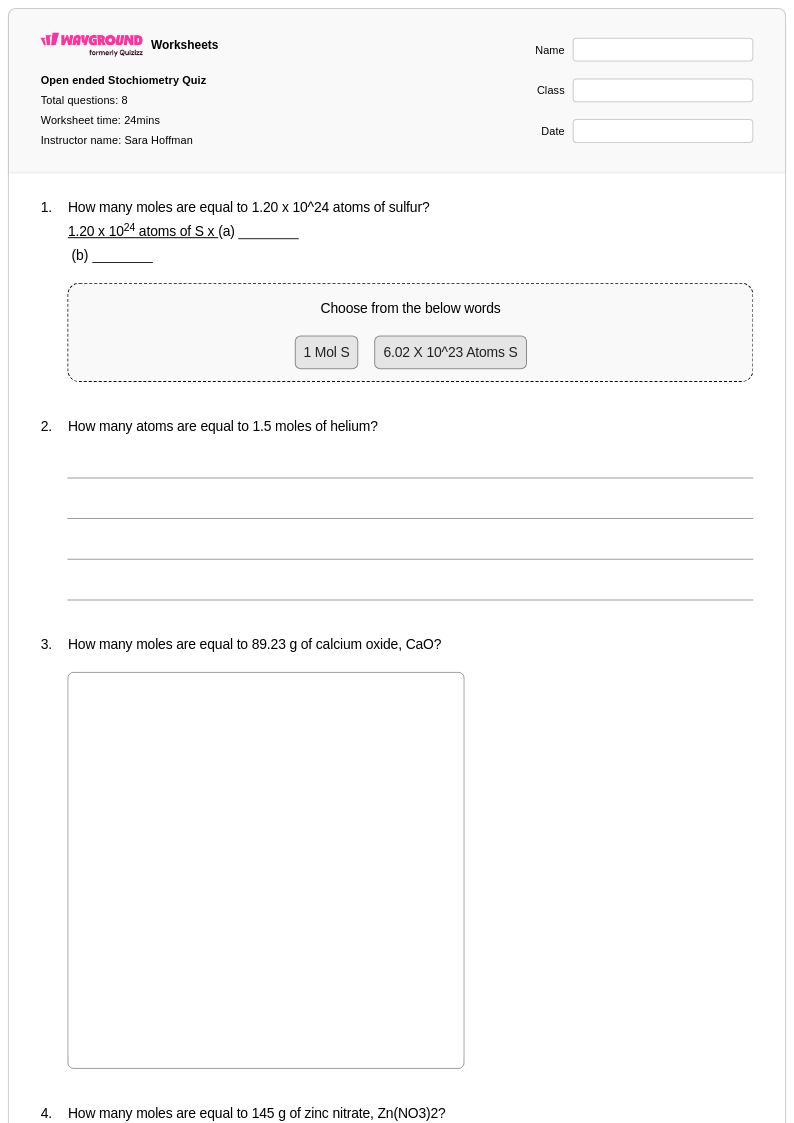
8 Q
9th - 12th

25 Q
10th - Uni

15 Q
12th - Uni
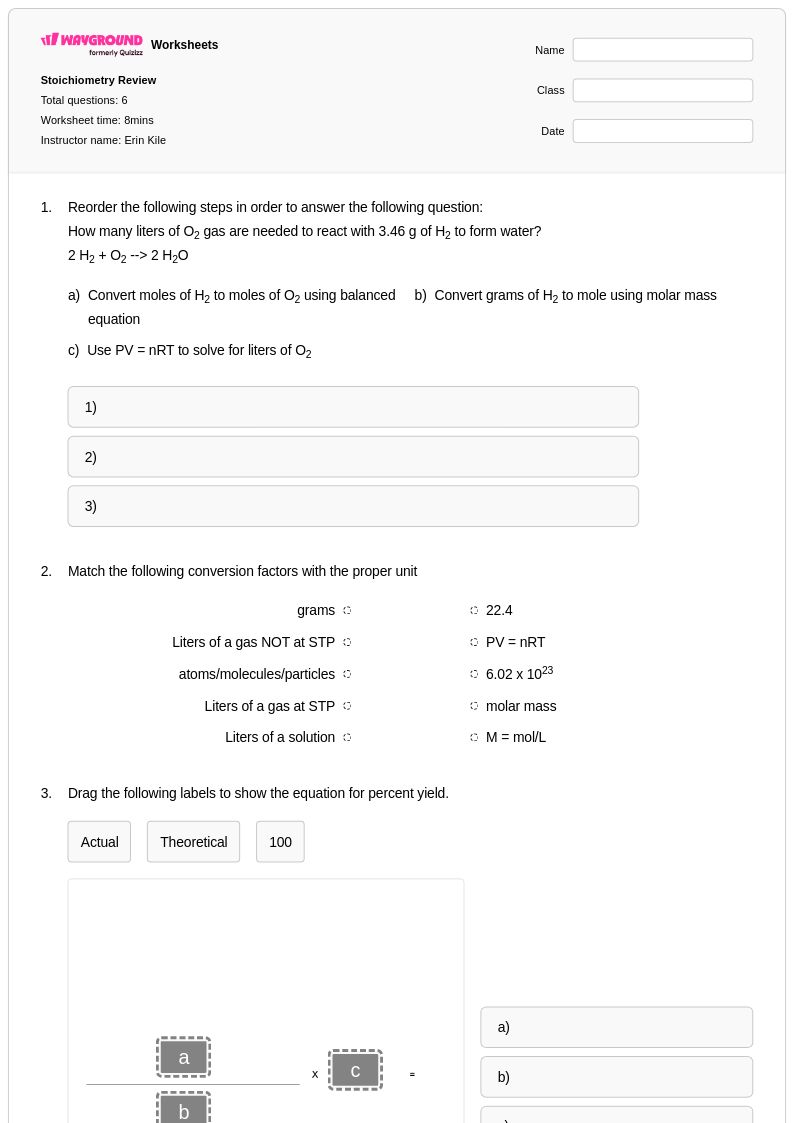
6 Q
9th - 12th

15 Q
9th - 12th
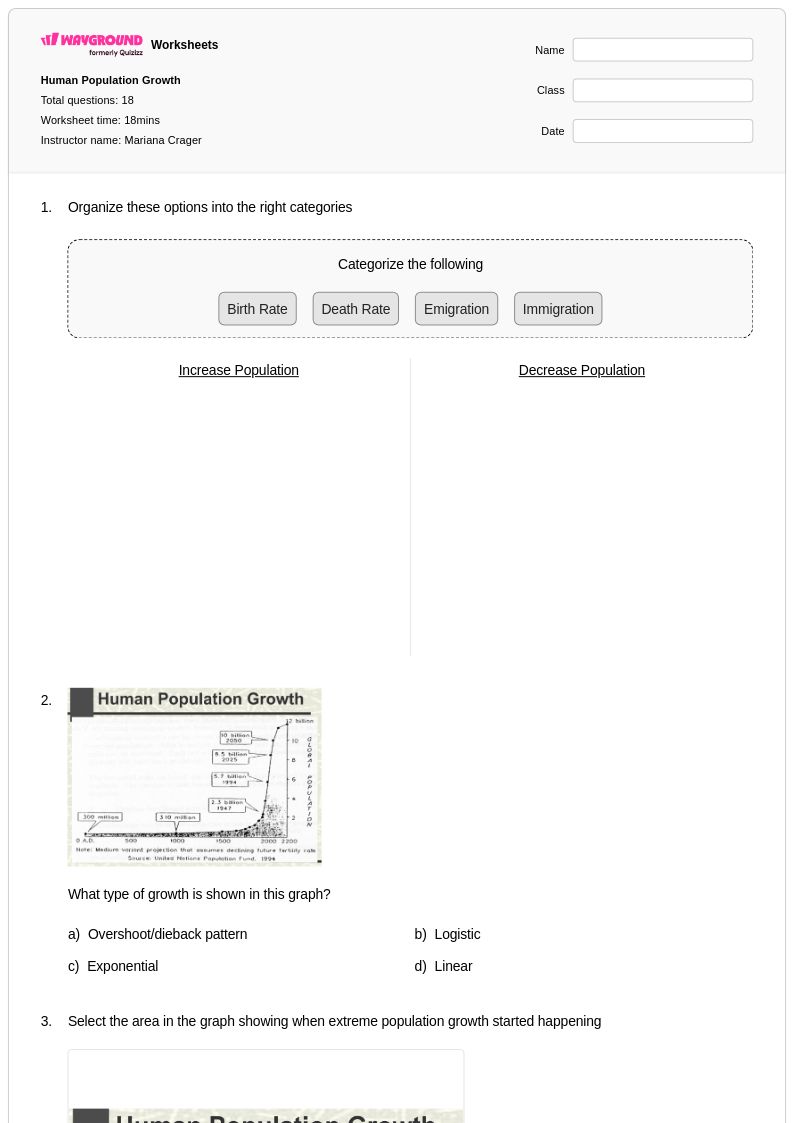
18 Q
12th
11 Q
10th

20 Q
8th
26 Q
5th

10 Q
8th

40 Q
7th
13 Q
6th

21 Q
KG
38 Q
6th

20 Q
7th
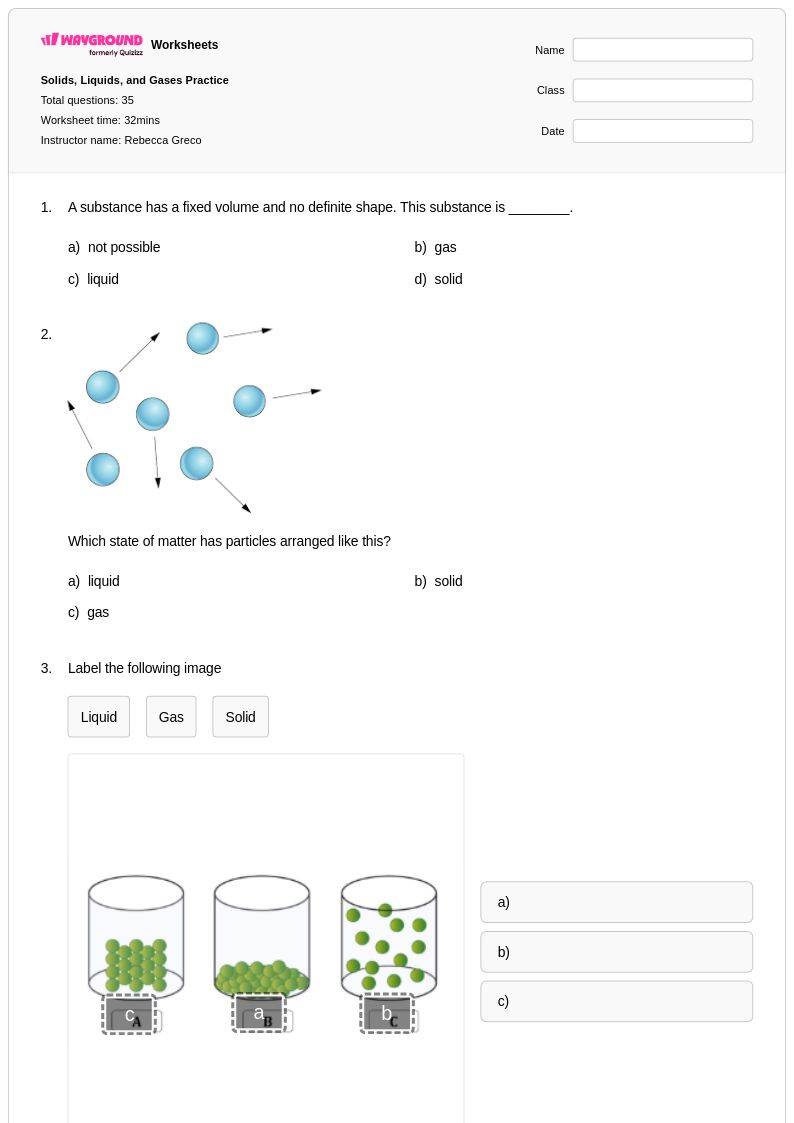
35 Q
6th
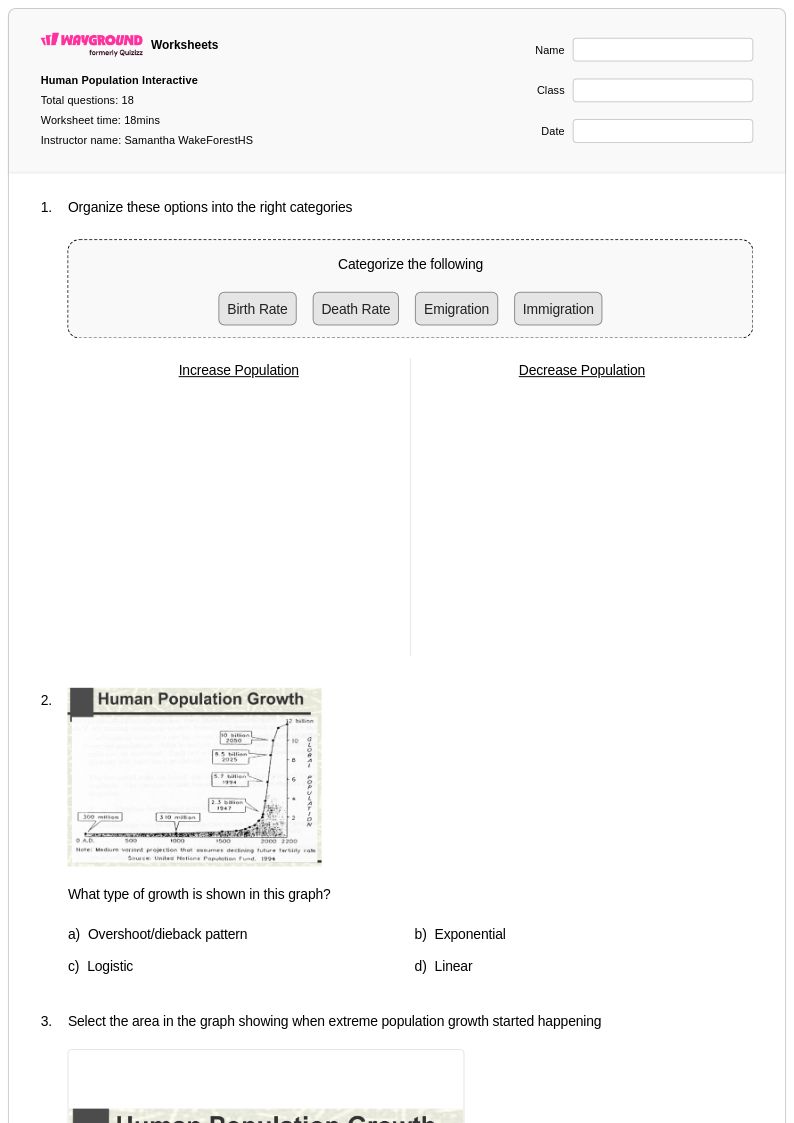
18 Q
11th
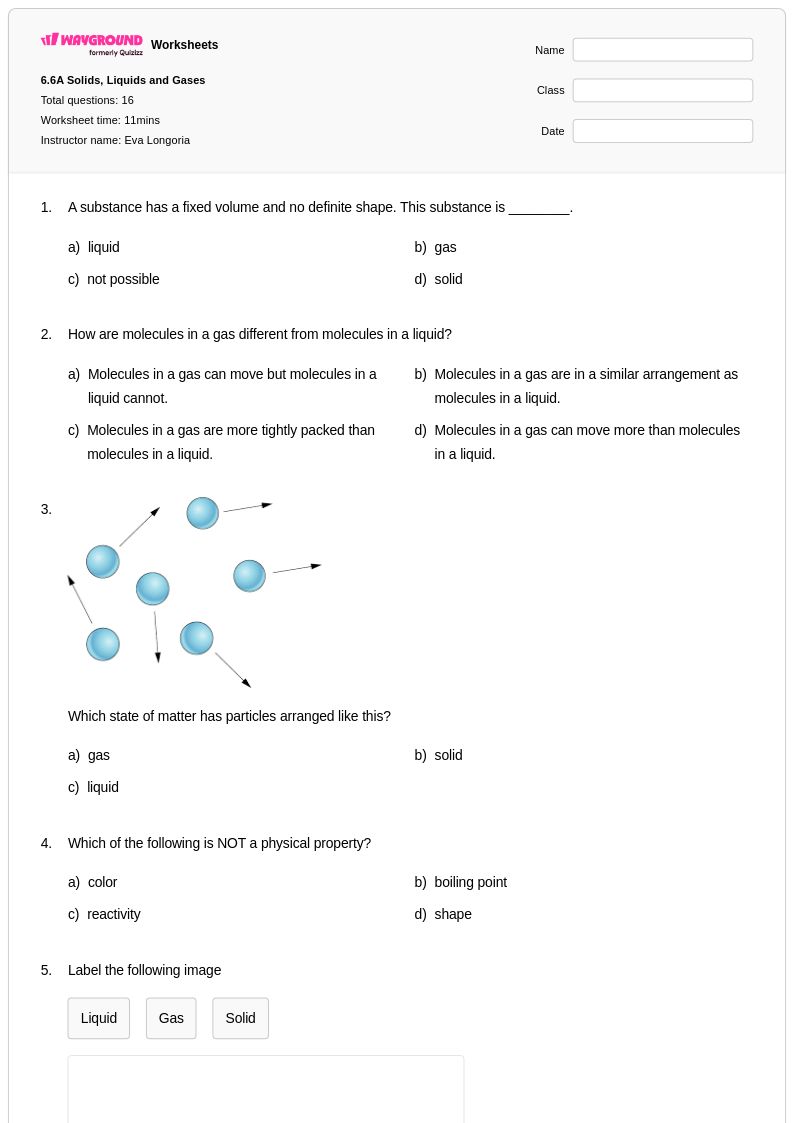
16 Q
6th

15 Q
6th

15 Q
11th - Uni

17 Q
7th
Explore Worksheets by Subjects
Explore printable Stoichiometry of Gases worksheets
Stoichiometry of gases worksheets available through Wayground (formerly Quizizz) provide comprehensive practice materials that strengthen students' ability to apply quantitative relationships to gaseous reactants and products in chemical reactions. These expertly crafted resources focus on essential skills including mole-to-mole conversions involving gases, calculating volumes at standard temperature and pressure, applying the ideal gas law to stoichiometric problems, and determining limiting reactants when gases are involved in chemical processes. The collection includes detailed practice problems that guide students through complex multi-step calculations, complete answer keys for immediate feedback and self-assessment, and free printables that reinforce conceptual understanding of how the molar volume of gases relates to balanced chemical equations.
Wayground (formerly Quizizz) empowers chemistry educators with millions of teacher-created stoichiometry of gases resources that streamline lesson planning and support diverse learning needs in the classroom. The platform's robust search and filtering capabilities allow teachers to quickly locate materials aligned with specific chemistry standards and learning objectives, while built-in differentiation tools enable customization of problem complexity to match individual student readiness levels. These comprehensive worksheet collections are available in both printable pdf formats for traditional paper-based practice and interactive digital versions that provide instant feedback, making them invaluable for targeted skill practice, remediation of challenging gas law concepts, and enrichment activities that challenge advanced learners to master sophisticated stoichiometric calculations involving gaseous systems.
