43 Q
6th - 8th
12 Q
8th

71 Q
11th
50 Q
9th - 12th
18 Q
11th - Uni

20 Q
10th

12 Q
8th

55 Q
9th - 12th
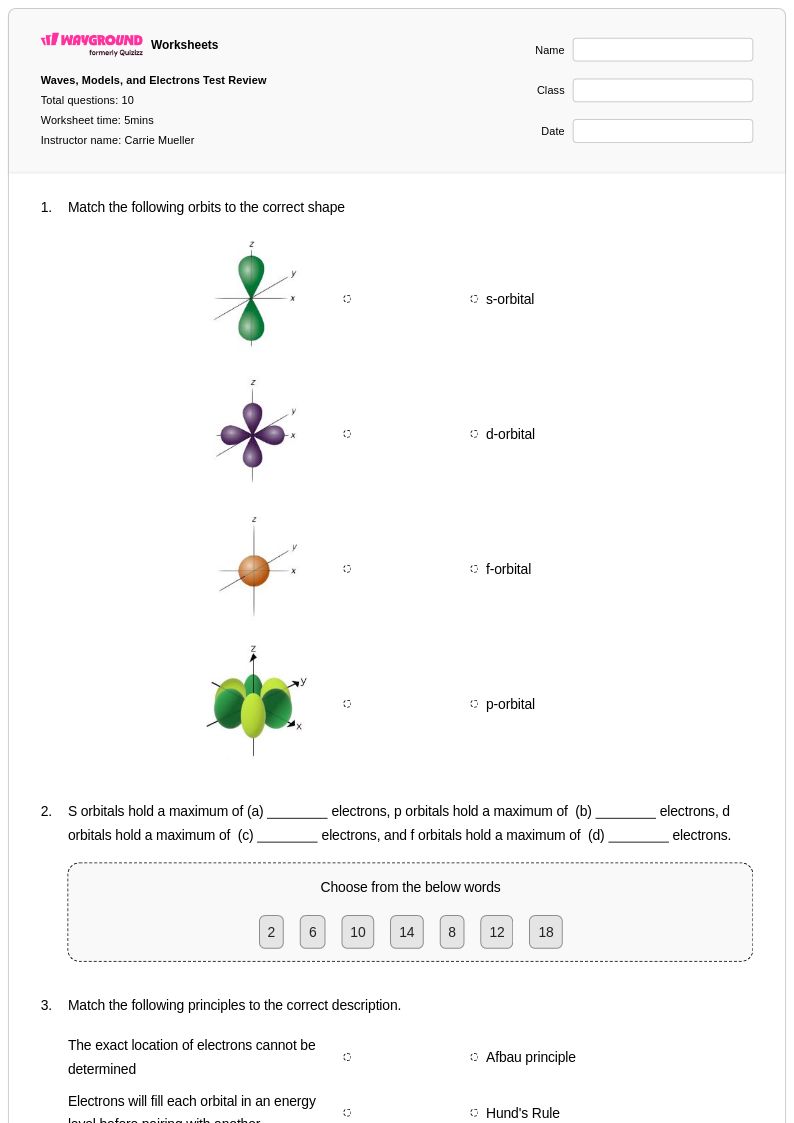
10 Q
10th

222 Q
11th

48 Q
9th
164 Q
12th
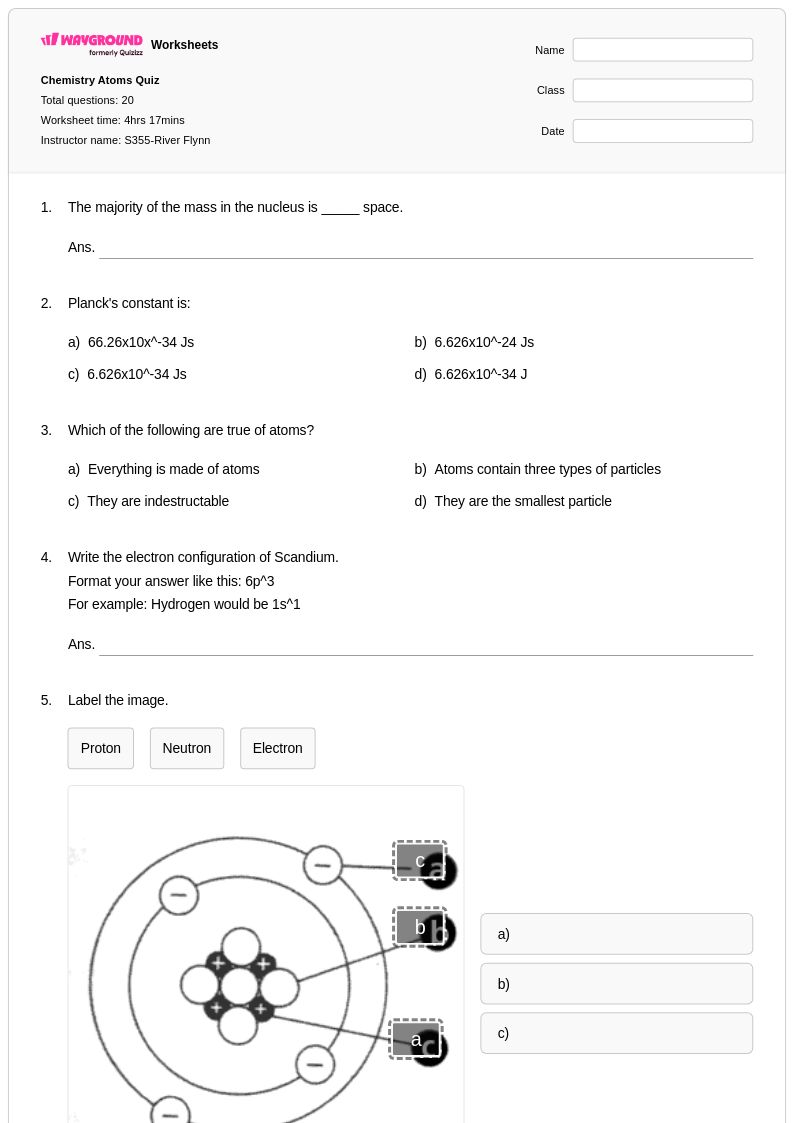
20 Q
10th
25 Q
10th
20 Q
10th - Uni
40 Q
9th

7 Q
12th
10 Q
8th

83 Q
10th

55 Q
10th

70 Q
12th
21 Q
9th - 12th

44 Q
10th

30 Q
11th
Explore Worksheets by Subjects
Explore printable Atomic Orbital Diagram worksheets
Atomic orbital diagram worksheets available through Wayground (formerly Quizizz) provide comprehensive practice opportunities for students to master the visualization and understanding of electron configurations in atoms. These expertly designed worksheets strengthen critical skills including drawing orbital diagrams using arrows to represent electron spin, applying Hund's rule for electron placement within subshells, and understanding the relationship between quantum numbers and orbital shapes. Students work through systematic practice problems that progress from simple hydrogen atom configurations to more complex multi-electron atoms, with each worksheet including detailed answer keys that explain the step-by-step process for constructing accurate orbital diagrams. The free printables cover essential concepts such as aufbau principle application, orbital energy level ordering, and the distinction between orbital notation and electron configuration notation, ensuring students develop both conceptual understanding and practical diagramming skills.
Wayground (formerly Quizizz) empowers educators with an extensive collection of teacher-created atomic orbital diagram resources, drawing from millions of high-quality worksheets that have been classroom-tested and refined. The platform's robust search and filtering capabilities allow teachers to quickly locate materials that align with specific learning objectives and standards, while built-in differentiation tools enable customization based on individual student needs and skill levels. These orbital diagram worksheets are available in both printable pdf formats for traditional classroom use and digital formats for interactive learning environments, providing maximum flexibility for lesson planning and implementation. Teachers can seamlessly integrate these resources into their curriculum for initial skill development, targeted remediation of electron configuration misconceptions, or enrichment activities that challenge advanced students to tackle complex transition metal and lanthanide orbital arrangements.
