
20 Q
6th
20 Q
9th - 12th

26 Q
9th
25 Q
9th - 12th

9 Q
2nd
20 Q
8th

26 Q
9th - Uni

41 Q
9th

12 Q
8th
23 Q
9th

16 Q
8th

15 Q
6th

17 Q
9th
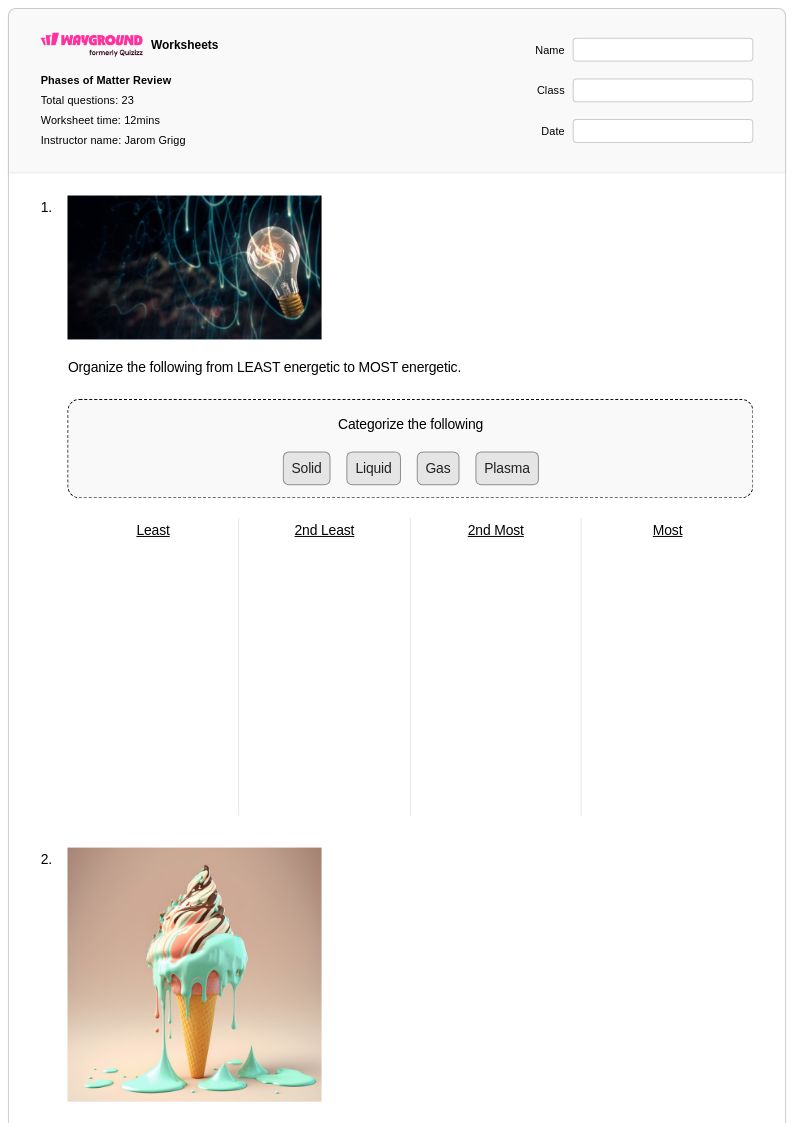
23 Q
8th
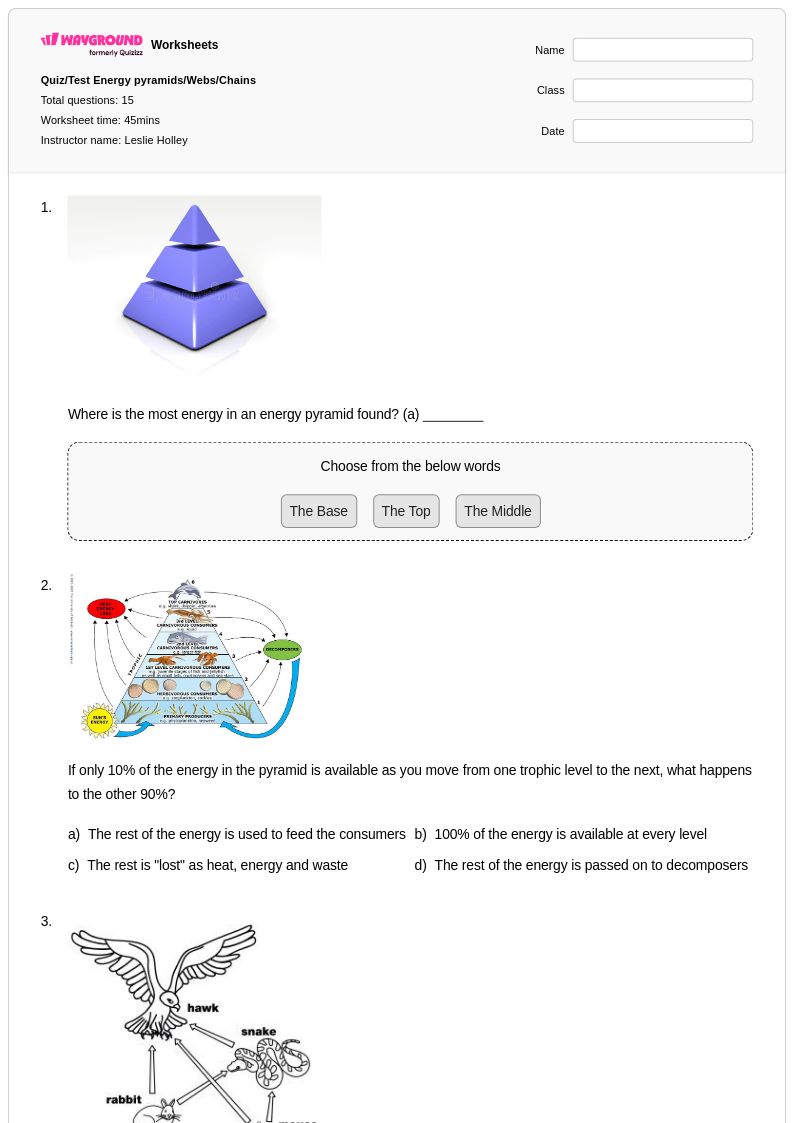
15 Q
8th
16 Q
8th

14 Q
7th
11 Q
7th

19 Q
7th
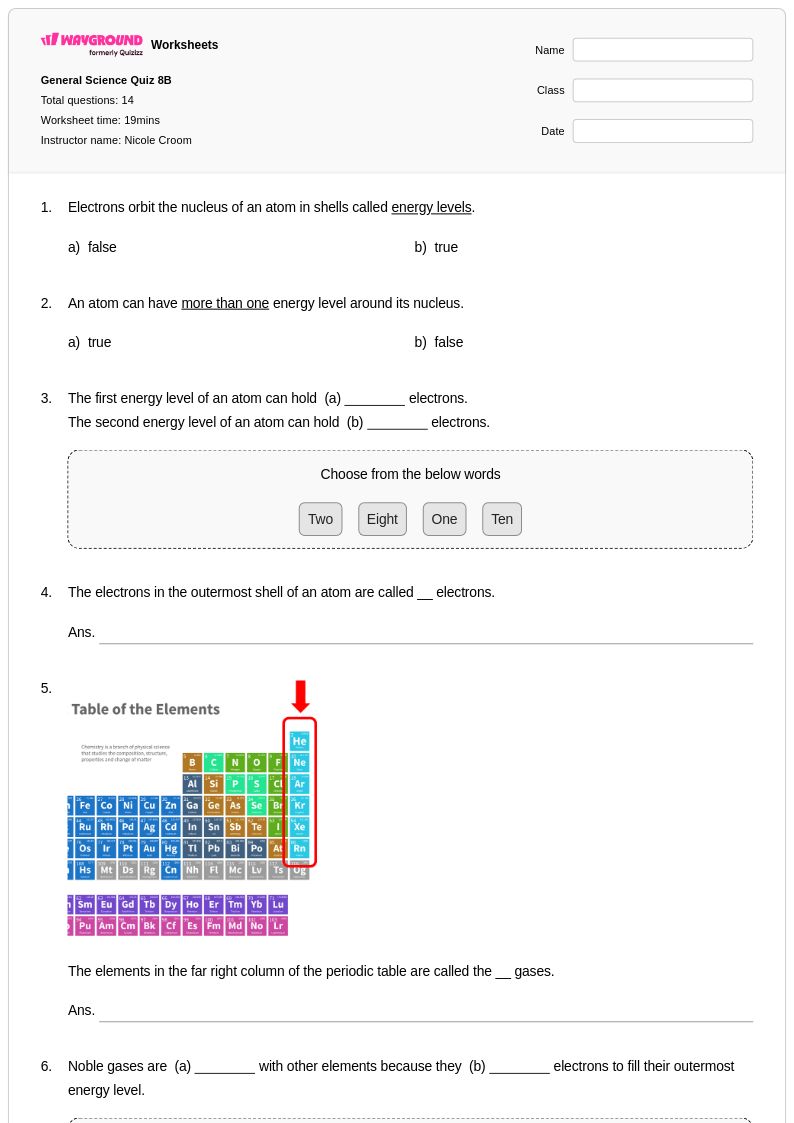
14 Q
8th
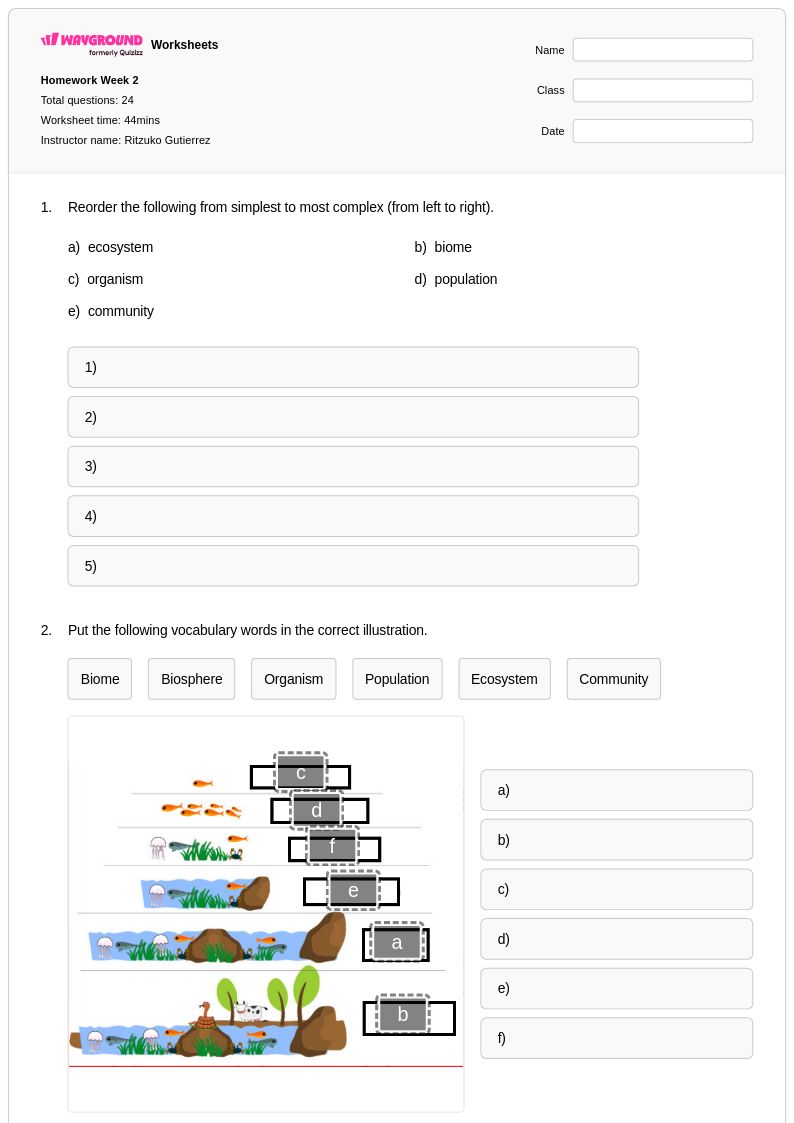
24 Q
12th

21 Q
12th

26 Q
8th

46 Q
10th
Explore Worksheets by Subjects
Explore printable Energy Levels worksheets
Energy levels worksheets available through Wayground (formerly Quizizz) provide comprehensive practice materials that help students master the fundamental concepts of electron configuration and atomic structure in chemistry. These carefully designed resources strengthen critical skills including identifying energy shells, understanding electron capacity within orbitals, and applying the aufbau principle to determine electron arrangements. Students work through practice problems that cover everything from basic energy level diagrams to complex electron configurations for transition metals, with each worksheet including a detailed answer key to support independent learning. The free printable materials are structured to build conceptual understanding progressively, allowing students to practice calculating ionization energies, predicting chemical behavior based on electron arrangements, and connecting energy levels to periodic trends.
Wayground (formerly Quizizz) empowers teachers with millions of teacher-created energy levels resources that can be easily searched, filtered, and customized to meet diverse classroom needs. The platform's robust collection includes materials aligned with state and national chemistry standards, offering both printable pdf formats and interactive digital versions that accommodate different learning preferences and technological capabilities. Teachers can efficiently differentiate instruction by selecting worksheets that target specific skill levels, from introductory energy shell concepts for beginning chemistry students to advanced quantum mechanical principles for honors classes. These flexible tools streamline lesson planning while providing targeted practice for remediation, skill reinforcement, and enrichment activities, ensuring that all students develop a solid foundation in atomic theory and electron behavior that supports their broader chemistry education.
